422177
Ethyl vinyl ether
contains 0.1% KOH as stabilizer, 99%
Synonym(s):
Ethoxyethylene
About This Item
Recommended Products
Quality Level
Assay
99%
form
liquid
contains
0.1% KOH as stabilizer
0.1% potassium hydroxide as stabilizer
refractive index
n20/D 1.376 (lit.)
bp
33 °C (lit.)
mp
−116 °C (lit.)
density
0.753 g/mL at 25 °C (lit.)
storage temp.
2-8°C
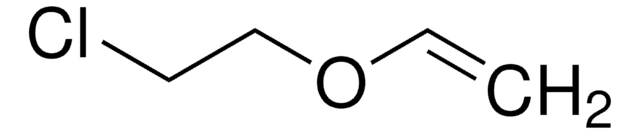
SMILES string
CCOC=C
InChI
1S/C4H8O/c1-3-5-4-2/h3H,1,4H2,2H3
InChI key
FJKIXWOMBXYWOQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Ethyl vinyl ether can be used as:
- A monomer to synthesize amphiphilic block copolymers to fabricate protein-repelling polymersomes to produce spherical nanoparticles. These nanoparticles can be used as drug carriers.
- A precursor to synthesize polymer electrolytes and cathode materials for solid-state lithium-ion batteries via UV photopolymerizations.
- A monomer to synthesize poly(vinyl ether)s with controlled molecular weight and narrow dispersity via photoinduced free radical-promoted cationic reversible addition–fragmentation chain transfer (RAFT) polymerization. These polymers are used to fabricate 3D objects with different thicknesses by employing stereolithography-based 3D printing.
- H-bonded Reusable Template Assisted para-Selective Ketonisation: This study discusses the use of ethyl vinyl ether in catalytic processes involving palladium and hexafluoroisopropanol to achieve para-selectivity in ketonisation, relevant for synthesizing complex organic compounds used in pharmaceuticals and materials science (A Maji, A Dahiya, G Lu, T Bhattacharya, 2018).
- Mechanistic Insight into Photocontrolled Cationic Polymerization: Explores the photocontrolled polymerization of ethyl vinyl ether, providing valuable knowledge for the development of light-responsive materials, which could have applications in drug delivery and smart material systems (Q Michaudel, T Chauviré, V Kottisch, 2017).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Flam. Liq. 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
-49.0 °F
Flash Point(C)
-45 °C
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service