All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8N2
CAS Number:
Molecular Weight:
144.17
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
bp
142 °C/15 mmHg (lit.)
mp
13 °C (lit.)
density
1.14 g/mL at 25 °C (lit.)
SMILES string
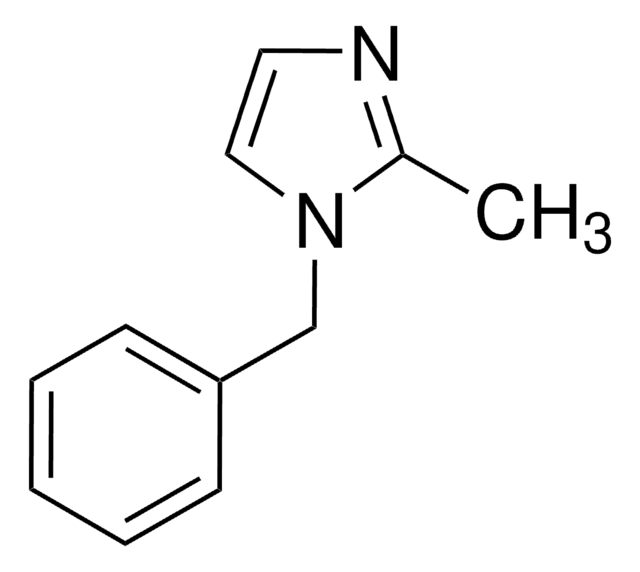
c1ccc(cc1)-n2ccnc2
InChI
1S/C9H8N2/c1-2-4-9(5-3-1)11-7-6-10-8-11/h1-8H
InChI key
SEULWJSKCVACTH-UHFFFAOYSA-N
General description
1-Phenylimidazole is an imidazole derivative. It induces 7-ethoxyresorufin-O-deethylase (EROD) activity in rainbow trout (Oncorhynchus mykiss) hepatocytes. The S(1)→S(0) transition of 1-phenylimidazole has been investigated in a supersonic jet expansion by resonant two-photon ionization. 1-Phenylimidazole is reported to be inhibitor of calmodulin-dependent nitric-oxide synthase from bovine brain and GHs pituitary cells.
Application
1-Phenylimidazole is a suitable reagent used to investigate its effect on the citrulline formation by bovine brain nitric-oxide synthase.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J A Zijlstra et al.
Mutation research, 198(1), 73-83 (1988-03-01)
The route of administration of a drug is a pharmacological factor to be reckoned with. In Drosophila, a whole-animal object for mutagenicity studies, the way in which a mutagen is applied can also be of crucial importance. In this study
Priyadarshini Balaraman et al.
Biochimica et biophysica acta. General subjects, 1863(2), 304-312 (2018-11-06)
The camphor-degrading microorganism, Pseudomonas putida strain ATCC 17453, is an aerobic, gram-negative soil bacterium that uses camphor as its sole carbon and energy source. The genes responsible for the catabolic degradation of camphor are encoded on the extra-chromosomal CAM plasmid.
Active-site structure analysis of recombinant human inducible nitric oxide synthase using imidazole.
R M Chabin et al.
Biochemistry, 35(29), 9567-9575 (1996-07-23)
Nitric oxide synthase catalyzes the pyridine nucleotide-dependent oxidation of L-arginine to nitric oxide and L-citrulline. It is a specialized cytochrome P450 monooxygenase that is sensitive to inhibition by imidazole. Steady-state kinetic studies on recombinant human inducible nitric oxide synthase (rH-iNOS)
Evan G Robertson et al.
The Journal of chemical physics, 121(24), 12421-12427 (2004-12-21)
The S(1)<--S(0) transition of 1-phenylimidazole (1PI) has been studied in a supersonic jet expansion by resonant two-photon ionization. The origin band at 36 075 cm(-1) is accompanied by a low frequency progression associated with torsion about the bond connecting phenyl
A R Barros et al.
Mutation research, 321(3), 119-126 (1994-05-01)
In order to investigate the role of metabolism in acrolein genotoxicity in D. melanogaster, the action of several metabolism modifiers, namely phenobarbital, an inducer of xenobiotic metabolism, phenylimidazole and iproniazid, inhibitors of oxidative activities of cytochrome P450, and diethyl maleate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service











![1-[2-(Trifluoromethyl)phenyl]imidazole](/deepweb/assets/sigmaaldrich/product/structures/150/780/ea7e6b25-7659-422e-868c-8df7fd70d66e/640/ea7e6b25-7659-422e-868c-8df7fd70d66e.png)

