179485
4-Oxo-TEMPO
Synonym(s):
4-Oxo-2,2,6,6-tetramethyl-1-piperidinyloxy, free radical
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H16NO2
CAS Number:
Molecular Weight:
170.23
EC Number:
MDL number:
UNSPSC Code:
12352000
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
storage temp.
2-8°C
SMILES string
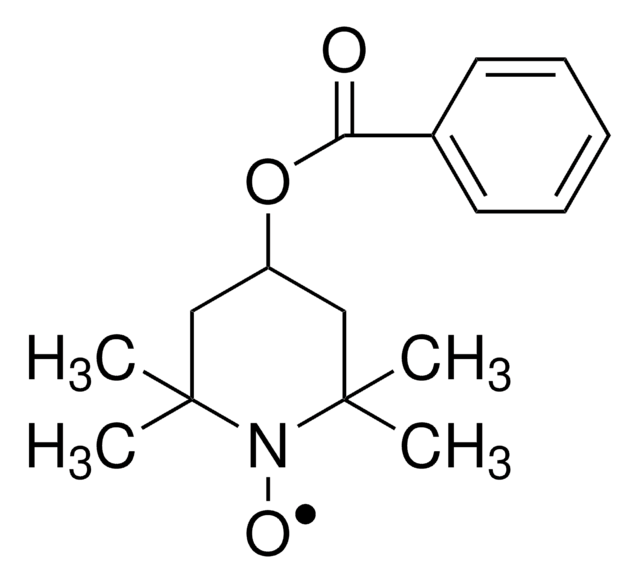
CC1(C)CC(=O)CC(C)(C)N1[O]
InChI
1S/C9H16NO2/c1-8(2)5-7(11)6-9(3,4)10(8)12/h5-6H2,1-4H3
InChI key
WSGDRFHJFJRSFY-UHFFFAOYSA-N
General description
4-Oxo-TEMPO is formed by the reaction of 2,2,6,6-tetramethyl-4-piperidone (4-oxo-TMP) and 1O2. It is a stable paramagnetic product formed during the irradiation of TiO2 in aqueous suspensions.
Application
4-Oxo-TEMPO was employed as nitroxide standard to investigate the generation of singlet oxygen by photoexcited TiO2 in ethanol by ESR spectroscopy. It may be employed as free radical polarizing agent in dynamic nuclear polarization (DNP) studies.
Free-radical nitroxide spin probe typically used for:
- Redox sources for anodes in lithium secondary batteries
- Free-radical biological studies
- Radical spin-trapping
- Electron paramagnetic resonance studies
- Polymer chemisty and synthesis applications
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Impact of Gd3+ on DNP of [1-13C] pyruvate doped with trityl OX063, BDPA, or 4-oxo-TEMPO.
Lumata L, et al.
The Journal of Physical Chemistry A, 116(21), 5129-5138 (2012)
Mark R Kurban et al.
The Journal of chemical physics, 129(6), 064501-064501 (2008-08-22)
Bimolecular collisions between perdeuterated 2,2,6,6-tetramethyl-4-oxopiperidine-l-oxyl molecules in three alkanes have been studied by measuring the electron paramagnetic resonance (EPR) spectral changes induced by spin exchange. We define an "encounter" to be a first-time collision followed by a series of re-encounters
Tsai-Mu Cheng et al.
Materials science & engineering. C, Materials for biological applications, 114, 111064-111064 (2020-10-01)
P-selectin overexpressed on activated endothelial cells and platelets is a new target for treatment of cancers and cardiovascular diseases such as atherosclerosis and thrombosis. In this study, depolymerized low molecular weight fucoidan (LMWF8775) and a thermolysin-hydrolyzed protamine peptide (TPP1880) were
R Konaka et al.
Redox report : communications in free radical research, 6(5), 319-325 (2002-01-10)
We previously reported that irradiation of titanium dioxide (TiO2) in ethanol generates both singlet oxygen (1O2) and superoxide anion (O2*-) as measured by EPR spectroscopy. The present study describes the production of reactive oxygen species upon irradiation of TiO2 in
Marina Bennati et al.
Physical chemistry chemical physics : PCCP, 12(22), 5902-5910 (2010-05-12)
Water (1)H relaxation rate measurements of (15)N-(2)H-TEMPONE solutions at temperatures ranging from 298 to 328 K have been performed as a function of magnetic field from 0.00023 to 9.4 T, corresponding to (1)H Larmor frequencies of 0.01 to 400 MHz.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)