All Photos(1)
About This Item
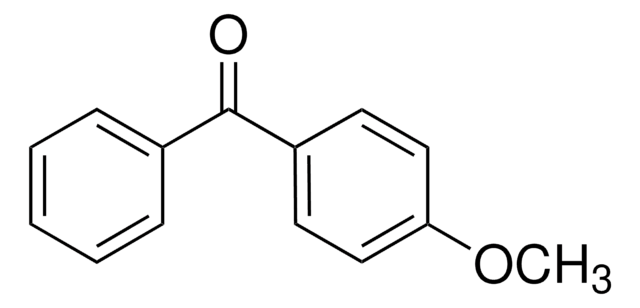
Linear Formula:
(CH3OC6H4)2CO
CAS Number:
Molecular Weight:
242.27
Beilstein:
1878026
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
141-143 °C (lit.)
SMILES string
COc1ccc(cc1)C(=O)c2ccc(OC)cc2
InChI
1S/C15H14O3/c1-17-13-7-3-11(4-8-13)15(16)12-5-9-14(18-2)10-6-12/h3-10H,1-2H3
InChI key
RFVHVYKVRGKLNK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Photoreactivity of 4,4′-dimethoxybenzophenone with 2-aminobenzimidazole has been examined. It is an ultraviolet absorbing additive in plastic products.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hirofumi Hirai et al.
Chemosphere, 55(4), 641-645 (2004-03-10)
Ligninolytic enzymes, manganese peroxidase (MnP), laccase, and lignin peroxidase (LiP), from white-rot fungi were used in an attempt to treat methoxychlor (MC), a chemical widely used as a pesticide. MnP and laccase in the presence of Tween 80 and 1-hydroxybenzotriazole
Daisuke Nakajima et al.
Journal of UOEH, 28(2), 143-156 (2006-06-20)
This study examines the activities relating to the carcinogenicity of six types of benzophenone derivatives (benzophenone, 2-hydroxy-4-octyloxybenzophenone, 2-hydroxy-4-methoxybenzophenone, 2,4-dihydroxybenzophenone, 2,2'-dihydroxy-4-methoxybenzophenone and 2,2'-dihydroxy-4,4'-dimethoxybenzophenone) currently used in plastic products as additives to serve as ultraviolet absorbing agents. The evaluation of the initiation
Xinggui Gu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(49), 17973-17980 (2015-10-23)
We present a nitrogen-containing polycyclic aromatic hydrocarbon (N-PAH), namely 12-methoxy-9-(4-methoxyphenyl)-5,8-diphenyl-4-(pyridin-4-yl)pyreno[1,10,9-h,i,j]isoquinoline (c-TPE-ON), which exhibits high quantum-yield emission both in solution (blue) and in the solid state (yellow). This molecule was unexpectedly obtained by a three-fold, highly regioselective photocyclodehydrogenation of a tetraphenylethylene-derived
Dolors Jornet et al.
The journal of physical chemistry. B, 114(36), 11920-11926 (2010-08-26)
This work has examined the photoreactivity of benzophenone (3), 2-benzoylthiophene (4), 4-methoxybenzophenone (5), 4,4'-dimethoxybenzophenone (6), and 4-carboxybenzophenone (7) with 2-aminobenzimidazole (1). Laser flash photolysis (LFP) revealed quenching of the aromatic ketone triplets by 1, leading to formation of ketyl radicals
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service