All Photos(2)
About This Item
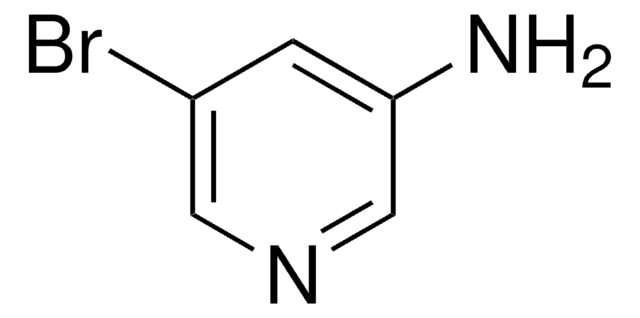
Empirical Formula (Hill Notation):
C5H5BrN2
CAS Number:
Molecular Weight:
173.01
Beilstein:
108737
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
133-138 °C (lit.)
SMILES string
Nc1ccc(Br)cn1
InChI
1S/C5H5BrN2/c6-4-1-2-5(7)8-3-4/h1-3H,(H2,7,8)
InChI key
WGOLHUGPTDEKCF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Amino-5-bromopyridine is a brominated aromatic amine reagent and is used for labeling of model reducing-end oligosaccharides via reductive amination.
Application
2-Amino-5-bromopyridine has been used to study the hydrogen-bonding patterns in the 2-amino-5-bromopyridine benzoic acid (1/1) cocrystal. It has been used in the synthesis of 2-amino-5-bromopyridinium 3-aminobenzoate salt and polycyclic azaarenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 48, 5039-5039 (2007)
Min Li et al.
Rapid communications in mass spectrometry : RCM, 17(13), 1462-1466 (2003-06-24)
Model reducing-end oligosaccharides were successfully labeled by a brominated aromatic amine reagent, 2-amino-5-bromopyridine (ABP), through reductive amination. Using either a combination of liquid chromatography/electrospray ionization mass spectrometry (LC/ESI-MS) with in-source fragmentation or liquid chromatography/electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS), sequence
Madhukar Hemamalini et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 3), o664-o664 (2010-01-01)
In the title salt, C(5)H(6)BrN(2) (+)·C(7)H(6)NO(2) (-), the pyridine N atom of the 2-amino-5-bromo-pyridine mol-ecule is protonated. In the crystal, the protonated N atom and the 2-amino group are hydrogen-bonded to the carboxyl-ate O atoms via a pair of N-H⋯O
Madhukar Hemamalini et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 3), o663-o663 (2010-01-01)
In the title adduct, C(5)H(5)BrN(2)·C(7)H(6)O(2), the carboxyl group of the benzoic acid mol-ecule is twisted away from the attached ring by 12.97 (11)°. The 2-amino-5-bromo-pyridine mol-ecules inter-act with the carboxylic group of neighbouring benzoic acid mol-ecules through N-H⋯O and O-H⋯N hydrogen
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)