EGFR-Family Receptor Dimerization Studies
Our aim was to investigate the dimerization and activation pattern of two receptors, EGFR (ErbB1) and HER2 (ErbB2), in the EGFR-family using breast cancer cell lines and selected colon tissues as model systems. The importance of the human epidermal growth factor receptor (EGFR) family in the development and progression of various cancers is widely recognized. Depending on the interaction partner for a specific receptor, different signaling cascades are activated. The Duolink® assay allows precise detection and quantification of proteins, protein interactions, and modifications in fixed cells and tissue samples in their correct cellular context at physiologically relevant expression levels.1 At least 11 different ligands are known to regulate receptor activation and dimerization.2 Here we give an example of how Duolink® assays can be used to study receptor dimerization and activation patterns in cells and tissue samples. Measuring these interaction patterns may have great potential as a companion diagnostic tool for the new generation anticancer therapies aimed at inhibiting specific receptor dimerizations.2
Duolink® Reagents and In Situ PLA Technology
The Duolink® kit series, developed by Olink Bioscience, allows the user to combine any pair of immunofluorescence or immunohistochemistry validated antibodies. Duolink® assay read-out is performed either with a fluorescent label for fluorescence microscopy or horseradish peroxidase for brightfield detection. The resulting distinct spots are derived from single protein-interaction events, which are visualized using a standard microscope.
Studying EGFR-Family Receptor Dimerization and Activation in Cells
To validate EGFR-HER2 dimerization and activation we used EGF, which exclusively binds and phosphorylates EGFR. Two different cell lines, SKBR3 (known to express EGFR and high levels of HER2) and MDA-MB-468 (known to express EGFR and very low levels of HER2) were stimulated with the ligand EGF. Both cell lines responded to EGF stimulation by phosphorylation of EGFR (Figure 1), which was measured using an anti-phospho-EGFR (pEGFR)(pTyr1068) antibody. However, the interaction between pEGFR and HER2 was detectable only in the SKBR3 cell line after EGF stimulation.
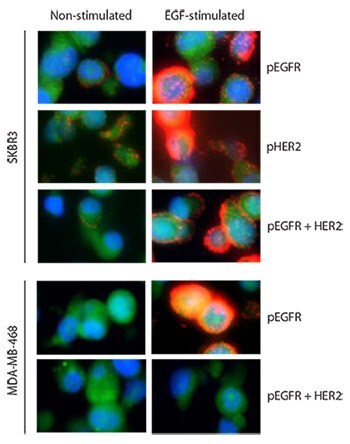
Figure 1.Two breast cancer cell lines, SKBR3 and MDA-MB-468, are assayed with Duolink® before and after EGF treatment for three targets: pEGFR alone, pHER2 alone, and the complex pEGFR-HER2. Red: PLA signals. Green: Sample autofluorescence (to visualize the cell morphology). Blue: nuclei (DAPI). Images were taken pair wise with the same exposure time of EGF-stimulated and non-stimulated samples for each assayed analyte.
This was expected, given the MDA-MB-468 cell line has very low levels of HER2 expression. To further confirm that EGFR-HER2 interactions took place, phosphorylated HER2 (pHER2) was measured using an anti-phospho-HER2 (pTyr1221/1222) antibody. EGF does not bind as a ligand to HER2; therefore, HER2 can be activated after EGF stimulation only via a complex with EGF-stimulated EGFR. The result showed a strong phosphorylation of HER2 in SKBR3. The use of an antibody targeting pEGFR instead of total EGFR ensured it was an activated EGFR-HER2 complex that was measured and not an inactive, preformed complex that does not elicit downstream signaling.3,4 The cells were formalin fixed and paraffin embedded.
Studying Tyrosine Kinase Receptor Dimerization and Activation in Tissue
Duolink® assays validated on cells (Figure 1) were transferred and applied directly to tissue samples, one case of serrated adenoma from colon and one case of colon primary tumor. Both cases were known to contain high levels of pEGFR (Figure 2A and 2B) and were assayed for pEGFR-HER2 complexes using the same assay as for the cells (Figure 1). Of these two samples, only the serrated adenoma exhibited high levels in the pEGFR-HER2 assay. A complex between activated EGFR and HER2 should lead to phopshorylation also of the HER2 receptor. The Duolink® assay measuring pHER2 presented high levels of pHER2 in the same tissue compartment in serrated adenoma that displayed high levels of pEGFR-HER2 complexes (Figure 2E). The primary tumor had low levels of the pEGFR-HER2 complex (Figure 2D) and displayed low levels of pHER2 (Figure 2F). The formalin fixed and paraffin embedded tissue were anonymized, only the pathological anatomical diagnoses were known.
Another example of how Duolink® assays can be used to study EGFR-family interactions was recently published for the HER2-PTK6 complex detected in FFPE material.5 The result indicates prognostic relevance of the HER2-PTK6 complex, showing the importance of investigating protein-interaction events for diagnostic use.
Conclusions
Duolink® reagents offer a unique opportunity to decipher EGFR-family receptor interactions. These hold great promise to further fine-tune companion diagnostics for EGFR-family receptor targeted therapies as well as a means to measure the effects of new drugs targeting specific interactions. In situ PLA, using Duolink® reagents, is a straightforward process for reporting protein interactions, including homodimerization, with very high specificity in their natural context at physiological expression levels. This enables an efficient and simple way for both validation and characterization of protein interactions. Duolink® PLA probes ensure any immunofluorescence or immunohistochemistry validated antibody can be used to fit the unique application of the user.
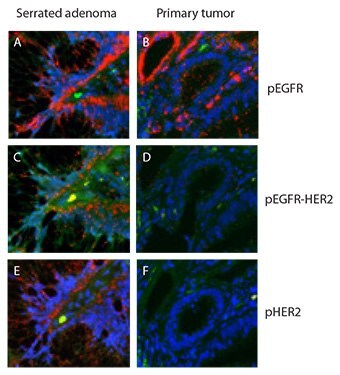
Figures 2A-F.Duolink® assay of HER-family dimerization and activation in colon tissue samples. Both samples contained high levels of pEGFR (A,B) but only the serrated adenoma showed high levels of pEGFR‑HER2 complexes (C,D) and of pHER2 (E,F). Red: PLA signals. Green: Sample autofluorescence (to visualize the cell morphology). Blue: nuclei (DAPI).
References
如要继续阅读,请登录或创建帐户。
暂无帐户?