Ultrapure Water for Metal Analysis in Regulated Food & Beverage Labs
Merina Corpinot (PhD)1, Lionel Lumet3, Estelle Riche (PhD)2, Gabriela Dima (PhD)1, Jean-Christophe Royer2
1R&D, Lab Water Solutions, Merck, Guyancourt, France, 2Strategic Marketing, Lab Water Solutions, Merck, Guyancourt, France, 3Environmental and Food and Beverage Testing Laboratory of Vendée, La-Roche-sur-Yon, France

Section Overview
- Elemental analysis of food and water samples
- Study: Quality control of food and bottled drinking water using Milli-Q® ultrapure water
- Analysis of elements in food samples
- Analysis of elements in bottled drinking water samples
- Conclusion: Role of ultrapure water in accurate multi-elemental analysis
- Related Products
Multi-elemental analysis, including ICP-MS (inductively coupled plasma–mass spectrometry) and ICP-OES (inductively coupled plasma–optical emission spectroscopy), is one of the most common analytical methods used to monitor environmental contamination and control food quality in order to assess health risk. Specific methodologies have been developed to reliably separate and detect regulated (and non-regulated) elements in food and water samples, in one single analytical run.
Elemental analysis of food and water samples
Health risks of elements in food and water
Environmental concentrations of elements are continuously increasing due to agricultural, industrial and other human activities. Studies reveal that some metals (e.g., As, Cd, Hg and Pb) show toxicity (such as neurological, cardiovascular, dermatological, hepatic, nephrotic, immunological and reproductive), while others provide nutritional benefits (Cu, Co, Fe, K, Mn, Zn).1,2 The health risks related to toxic metals depend on their concentration and the length of exposure. Several parts of the world are impacted by drinking water3 and food contamination.4 For this reason, regulations are evolving to allow the effective and standardized measurement of these toxic chemicals.
Regulation of elemental contamination in food and water
Maximum levels of elemental contaminants in foodstuffs and drinking water have been established to protect human health and the environment. Multi-elemental analyses help to achieve compliance with local, national and international guidelines. Standards for the quality of water are set by the World Health Organization (WHO)5 and regulations are implemented by individual countries.
In Europe and in the US, the EU Drinking Water Directive6 and the US Safe Drinking Water Act (Environment Protection Agency),7 respectively, establish standards to ensure the quality of tap water and set methods to monitor levels of contaminants.
With respect to food products, the EU Commission’s Regulation (EC) No. 1881/20068,9 used to set acceptable levels for Cd, Hg, Pb and inorganic Sn. The US FDA gives guidelines to industry for As and Pb,10 and, in 2021, the FDA started the Closer to Zero program to target a larger range of metals. The goal of this program is to reduce dietary exposure to contaminants to as low as possible, while maintaining access to nutritious foods, especially for babies and young children.
Requirement for high-purity water in testing labs
To respond to customer requirements, it is important for testing laboratories to use high-purity water to ensure precision and accuracy when measuring these critical elements. Water is used at each step of analyses done by ICP-OES or ICP-MS, including:
- As a reagent blank
- For sample and standard preparation
- For instrument and sample container cleaning
Laboratories must be confident in the high quality and the consistency of their purified water, as water contaminants can impede optimal instrument functioning, cause interferences, and impact results reliability.
Study: Quality control of food and bottled drinking water using Milli-Q® ultrapure water
We assessed the use of ultrapure water delivered from a Milli-Q® IQ 7000 water purification system for the multi-elemental analysis of various food and bottled drinking water samples. We chose food samples (tuna, rice, apple sauce, red meat and oysters) that are often suspected to be contaminated with toxic metals. Bottled drinking water samples were chosen knowing that water analysis is submitted to regulations. Quality control was performed according to EU regulations.8,9
Analytical methods and materials
Regulated elements
To evaluate compliance with EU requirements for toxic elements (Cd, Cr, Hg, Pb, Sn), validated methods were used to analyze food samples commonly tested by food and environmental testing laboratories. Although no regulatory limits are set for other heavy metals at the European level, France regulatory bodies set notification levels for certain metals (Al, Co, Cr, Ni, Se, Zn) to control food safety and quality (such as contamination, authenticity and traceability of products).
ICP-MS analysis & instrument parameters
Analyses were performed by the accredited Environmental and Food and Beverage Testing Laboratory in Vendée, France.11 The laboratory performed multi-elemental analyses of food and bottled drinking water samples using ICP-MS. Each sample was analyzed three times, and the results were expressed as means, standard deviations and relative standard deviations.
- Food samples were analyzed according to the French government initiative, the ANSES/LSAliments-LSA-0084 method of analysis for food safety12 (for As, Cd, Hg and Pb) or an internal ICP-MS method (based on ANSES/LSAliments-LSA-0084 method for Al, Cr, Co, Cu, Ni, Se, Sn, Zn).
- Bottled drinking water samples were analyzed following two different methods. Method 1 was based on Directive 2020/21846 regarding the quality of water intended for human consumption. The limits of detection (LOD, the lowest concentration that can be reliably detected using an analytical method) were evaluated and calculated according to the NF EN 17294-1-2004 norm.13 The limits of quantification (LOQ, the minimum concentration of the element that can be accurately measured using an analytical method) were set according to the decision of the 19/10/2017 decree14 and validated in the matrix according to the NF T 90-210 norm. Method 2, deriving from the NF EN 17294-1-2016 norm,15 was developed by the laboratory to analyze low levels of elements in water samples.
ICP-MS instrument parameters (Table 1) were selected to achieve a high level of sensitivity, reliability, accuracy and precision.
Chemicals & water source
Ultrapure water [18.2 MΩ.cm, total organic carbon (TOC) < 5 µg/L] delivered from a Milli-Q® IQ 7000 water purification system was used to prepare the samples and calibration solutions as described in the above-mentioned regulatory standards. The water system was equipped with a Millipak® filter at the point of dispense, which contains a 0.22 µm membrane filter to ensure removal of particles and bacteria. Ultrapure nitric acid was obtained by distillation. Standard solutions were purchased from Tech Lab.
Calibration solutions
Solutions for calibration were prepared from stock solutions by dilutions using freshly dispensed Milli-Q® ultrapure water and ultrapure nitric acid. These calibration solutions were used to make calibration curves and assess the linearity of the study range of the method.
Linear calibration curves were obtained
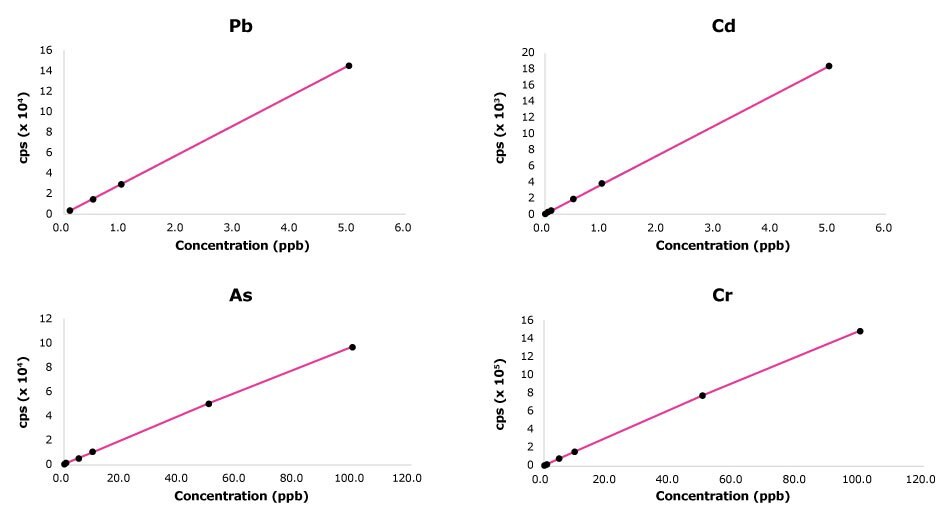
Figure 1.Examples of ICP-MS calibration curves for Pb, Cd, As and Cr used for the analysis of bottled drinking water samples.
The calibration range and LOQ for each element were established based on each element’s maximum levels set by EU regulatory bodies.6,8 At least five standard concentrations were used for each element. Examples of calibration curves for Pb, Cd, As and Cr in the context of the analysis of foodstuffs and drinking water samples are shown in Figure 1.
For the analysis of foodstuffs, the linearity of each standard curve was close to 1 for the tested elements. The LOD values ranged from 0.0002 to 0.004 mg/kg. The LOQ values ranged from 0.005 to 0.25 mg/kg.
For the analysis of water samples, the linearity of each standard curve was close to 1 for the tested elements.The LOD values ranged from 0.004 µg/L to 32 µg/L. The LOQ values ranged from 0.01 µg/L to 1000 µg/L.
High-purity water of consistent quality contributed to obtaining linear calibration curves. Preparing calibration standards and diluting samples with high-quality water ensures that only the elements analyzed are the ones intentionally added in the standard solutions, preventing elemental contamination that can lead to inaccurate results and errors in the calibration process. Overall, linear calibration curves help researchers to obtain more accurate and reliable results with greater sensitivity (lower detection limits).
Analysis of elements in food samples
Table 2 presents the quantitative elemental results for the food samples tested and the LODs for each element. All samples were found to be compliant with the regulations set by the French legislation. The study found that arsenic and mercury levels in food can vary widely from brand to brand, and be higher in organic than in conventional food, likely due to the cultivation environment. It is important to note that this study does not permit broad generalizations about food quality (because of limited representative sampling, limited random sampling, etc.); rather, its aim is to highlight the role of water in the performance of the analysis of elements.
To enable a comprehensive analysis of the elements of interest, it is crucial to minimize contaminant levels in the water used to perform the analysis. Our findings illustrate that the ultrapure water used in the analytical process facilitated our ability to reach low LODs and yield reliable outcomes.
Analysis of elements in bottled drinking water samples
Similarly, samples from two different bottled water brands were analyzed to quantitative levels of regulated and non-regulated elements according to EU regulations6 (Table 3). All concentrations of elements of concern, such as Pb, were below the WHO recommendations.
Leveraging a more sensitive method (i.e., ‘Method 2’, last column of Table 3), we further demonstrated that the levels of elements in Milli-Q® ultrapure water align with or even fall below the LODs calculated for the established method, Method 1. These results confirm the suitability of Milli-Q® ultrapure water for conducting sensitive analyses.
Conclusion: Role of ultrapure water in accurate multi-elemental analysis
When performing multi-elemental analysis, such as ICP-MS or ICP-OES, it is critical to use ultrapure water that is free of the elements being analyzed. The absence of metals and other impurities in Milli-Q® ultrapure water is necessary, as they can influence blanks, standards and sample preparation, instrument cleaning and cause interferences during analysis.
This investigation allowed to establish the suitability of Milli-Q® ultrapure water for the elemental analysis of food products and drinking water, essential for ensuring consumer health and product quality. Milli-Q® ultrapure water systems facilitate the daily operations of testing laboratories operating within standardized environments, ensuring consistent water quality for reliable, reproducible, and repeatable analytical results. Furthermore, the water purification systems uphold data traceability, a critical aspect for laboratories subjected to audits.
A range of water purification solutions are available tailored to meet the needs of scientists working with in environmental and food and beverage testing laboratories.
References
如要继续阅读,请登录或创建帐户。
暂无帐户?