Speeding Biosimilar Development using Glycosylation Control Technology from SAFC
The biosimilar market is expected to grow rapidly in the coming years, as many blockbuster monoclonal antibodies lose patent protection by 2020. In addition, with the availability of an approval pathway in the U.S., there are now opportunities for biosimilar manufacturers to enter major markets around the globe. In 2014, biosimilars accounted for just $2.0 billion of the $230 billion biopharmaceutical market, according to BCC Research, but revenues are growing at a compound annual growth rate of 15%. Meanwhile, Daedal Research estimates that there are 280 biosimilars in the pipeline, and the number of biosimilars in clinical trials is increasing at a rate of 20% per year.
Despite this strong growth, there will be stiff competition in the biosimilar market as numerous companies vie for market share. Only the first few to receive marketing approvals will establish sizable positions for any given biosimilar. Consequently, rapid product development will be crucial, and access to advanced technologies that can accelerate development timelines will provide biosimilar manufacturers with real competitive advantage.
Glycosylation Patterns Make a Difference
To receive approval, a biosimilar must have physicochemical/structural/functional properties that are comparable to those of the targeted branded biologic. Thus, not only must the physical and chemical characteristics of the biosimilar closely match those of the branded biologic, the pharmacodynamics, pharmacokinetics and immunogenicity of the biosimilar must be comparable to ensure similar efficacy and safety profiles.
Many peptides and proteins in mammalian cells are modified with sugar molecules. Consequently, therapeutic proteins are often subjected to glycosylation, a form of post-translational modification. The type, number and locations of sugar molecules, or the glycosylation pattern, of a protein affect not only its structure and folding ability, but its stability, in vivo halflife and transportability, immunogenicity and efficacy (Figure 1). Careful control of glycosylation chemistry during biosimilar manufacturing is therefore necessary to ensure optimal matching of glycosylation patterns and the ability to attain properties comparable to the targeted branded drug.
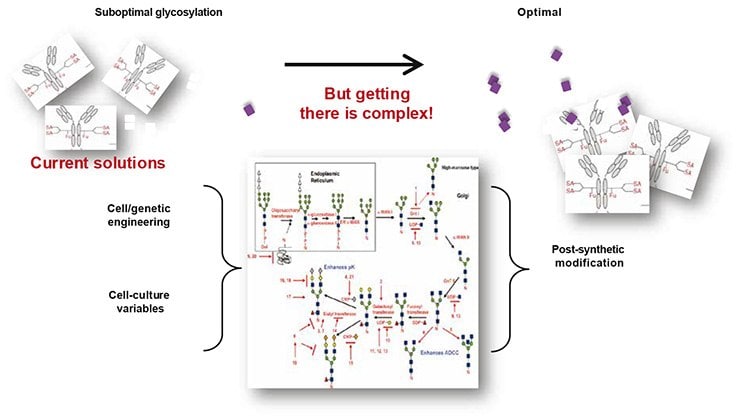
Figure 1: Poor glycosylation compromises the safety, pharmacokinetics and activity of biologic drugs.
Both the control of glycosylation patterns and glycan analysis can be challenging given the potential for complexity and diversity. Fortunately, recent advances in cell technology and high-throughput analytical techniques enable the efficient production of high-quality, glycosylated recombinant proteins and rapid determination of biosimilar comparability.
High Titers, Robust Scalability and Superior Protein Quality with the CHOZN® Platform
The CHOZN platform from SAFC is a Chinese hamster ovary (CHO) mammalian cell expression system for the fast and easy selection and scale-up of clones producing high levels of recombinant proteins. This complete cell line solution was specifically designed to accelerate development timelines and is ideal for the production of biosimilar proteins.
The cGMP manufactured cell lines are fully characterized, safety tested and regulatory compliant, ensuring secure biosimilar production. Because they are designed to be highly robust and productive as well as provide a high rate of clone recovery, fewer clones need to be screened, and there is no need for amplification. In fact, more than 70% of clones maintain more than 90% titer over 60 generations, and up to eight weeks of cell line development time can be eliminated through the use of the CHOZN mammalian cell expression systems (Figure 2).

Figure 2:CHOZN GS Clone Development Timeline
Comprehensive user protocols also provide guidance from transfection through small-scale bioreactor production, and the complete chemically defined process allows for seamless scaleup. As a result, it is possible to rapidly develop recombinant cell lines through strong metabolic selection and consistently obtain high titer, robust production clones.
Optimized Media and Feeds for Enhanced Performance
The CHOZN platform includes cGMP-produced, chemically defined media and feeds that have been optimized to maximize the growth and production of recombinant proteins from producing clones. Designed to address the need for faster speed to market, the EX-CELL® Advanced™ product line provides increased performance, streamlined regulatory compliance and supply chain security.
The chemically defined EX-CELL® Advanced CHO Fed-batch system affords improved production of many different proteinbased products, including mAbs and fusion proteins, in a wide range of industrial CHO cell lines, including SAFC’s CHOZN system. To develop the system, applied mathematics (multivariate analysis, data mining) was used to establish correlations of characterized raw materials to critical process and product attributes, such as doubling time, specific productivity, waste product formation and charge variance. As a result, three- to fivefold increases in therapeutic productivity have been achieved while maintaining critical protein attributes, and in the CHOZN platform cell line, the EX-CELL® Advanced system supports titers in excess of 5 g/L.

The improved performance of the EX-CELL® CHO Fed-batch system was also confirmed by evaluating the viable cell densities and titers obtained with new system and other leading commercially available CHO cell culture media and feed supplements from three different vendors for three distinct model CHO cell lines (CHO S, CHOZN GS and CHO dhr). An initial adaptation step was employed to select the top competitive media for each model cell line, and only those media successfully adapted within three passes were chosen for the study. In all instances, and regardless of cell bank medium, the cultures easily adapted to the EX-CELL® Advanced CHO Fedbatch media platform. All cultures were monitored for viable cell density and measured for antibody (IgG) titers up to day 14.
The EX-CELL® Advanced CHO Fed-batch platform demonstrated a sustained high biomass, with maximum titers across all model cell lines, and proved to be the only formulation with trouble-free adaptation from hydrolysate-containing or other chemically defined formulations (Figure 3). As important, the optimized media and feed combination outperformed the other leading commercial fed-batch products with a two- to fourfold increase in titers.
Directed Glycosylation with EX-CELL® Glycosylation Adjust (Gal+)
Most glycosylation processes involve reaction at the nitrogen group in the amino acid asparagine (N-glycosylation), but different enzymes and transporters are involved in different clones and cell lines, and even in different cells. The reaction media and reaction conditions can also affect the process. As a result, it can be very challenging to realize a target glycosylation pattern. EX-CELL® Glycosylation Adjust (Gal+) protein quality supplement was specifically developed by SAFC to help biosimilar manufacturers to achieve functionally relevant shifts in N-linked glycosylation quickly and with little effort.
The off-the-shelf GMP protein quality supplement, which is simply titrated into the bioreactor, allows users to easily achieve desired N-linked glycosylation by increasing the level of galactose site occupancy on the oligosaccharide. In doing so, months of trial-and-error optimization studies can be avoided, and both development time and costs can be significantly reduced.
EX-CELL® Glycosylation Adjust (Gal+) has been demonstrated to be effective in a broad range of cell lines, including the above-described CHOZN cell-line platform with the EX-CELL® Advanced CHO Fed-batch system and CHO-GS, CHO-M, DxB11 and NS0 lines. Supplemented fed-batch production processes have demonstrated two- to fourfold increase in relative G1F and G2F distributions when compared with processes without the addition of EX-CELL Glycosylation Adjust (Gal+) (Figure 4). The results for three different mAb-producing CHO cell lines are also presented in Table 1.

Figure 4:Directing glycosylation in the EX-CELL® Advanced Fed-batch system
Note: 14-day fed-batch culture performance with culture supplementation initiated at 0.2% (v/v) beginning on day 2 and then every other day.
Importantly, the desirable process outputs remained unaffected by the addition of the EX-CELL® Glycosylation Adjust (Gal+) protein quality supplement. In all three examples, identical or higher cell densities and volumetric productivities were obtained (Table 1). With this high level of cross-functionality, it is possible to rescue processes that suffer from poor glycosylation profiles and develop the data required for regulatory approvals, particularly for biosimilars.
Accelerate Development Times with Customized Support from SAFC
Support from SAFC does not end with product delivery. We have extensive capabilities in cell line engineering and development, formulation development and high-throughput analysis. Our mission is to facilitate our customers’ research and manufacturing projects through application of our experience and technology.
SAFC also leverages its CompoZr® Zinc Finger Nuclease technology to develop novel cell lines for customers, including CHO, with precise and heritable gene additions, deletions or modifications. In addition, we can develop customized media and/or feeds for optimal performance of customer host cell lines.
When combined with our high-throughput technology for rapid glycan analysis, these capabilities enable SAFC to quickly optimize customer platforms, and provide improved cell culture performance and enhanced product quality and efficacy through greater control of glycosylation, removal of interfering host cell proteins and creation of metabolic selection markers.
Conclusion
In the rapidly growing, but highly competitive biosimilar market, success will be awarded to those companies that are first to market. Biosimilar manufacturers with access to advanced technologies that provide for rapid development of optimized cell lines with excellent cell culture performance in terms of both productivity and glycosylation control will have a competitive advantage.
The CHOZN platform from SAFC, when combined with the EX-CELL® CHO Fed-batch system and EX-CELL® Glycosylation Adjust (Gal+) protein quality supplement, offers biosimilar producers a comprehensive solution specifically designed for accelerated development of biosimilars with high titers and cell viabilities as well as enhanced control over glycosylation profiles. These state-of-the-art technologies are also backed by comprehensive, customized support from SAFC throughout the biosimilar development process.
如要继续阅读,请登录或创建帐户。
暂无帐户?