Purification Using MBPTrap™ HP Columns
MBPTrap HP 1 mL and 5 mL columns (Figure 6.2) are made of biocompatible polypropylene that does not interact with biomolecules. Prepacked MBPTrap HP columns provide fast, simple, and easy separations in a convenient format. They can be operated with a syringe, a laboratory pump, or a liquid chromatography system such as ÄKTA.
MBPTrap HP columns are delivered with a stopper on the inlet and a snap-off end on the outlet. Porous top and bottom frits allow high flow rates. MBPTrap HP columns belong to the HiTrap family of prepacked columns. Note that HiTrap columns cannot be opened or refilled.
Table A3.2 (Appendix 3, Characteristics of Dextrin Sepharose High Performance products) summarizes the characteristics of prepacked MBPTrap HP columns.
Figure 6.2.MBPTrap HP 1 mL and 5 mL columns give fast and convenient affinity purifications of recombinant proteins tagged with MBP.
Sample Preparation
Adjust the sample to the composition of the binding buffer (see below). For example, dilute the sample with binding buffer or buffer exchange using a desalting column (Chapter 11, Desalting/buffer exchange and concentration).
Pass the sample through a 0.22 µm or a 0.45 µM filter and/or centrifuge it immediately before applying it to the column. If the sample is too viscous, to prevent it from clogging the column dilute it with binding buffer, increase lysis treatment (sonication, homogenization), or add DNase/RNase to reduce the size of nucleic acid fragments.
Water and chemicals used for buffer preparation should be of high purity. Filter buffers through a 0.22 µm or a 0.45 µm filter before use.
Purification
MBPTrap HP columns can be operated with a syringe, a laboratory pump, or a liquid chromatography system such as ÄKTA.
- Fill the syringe or pump tubing with binding buffer. Remove the stopper and connect the column to the syringe (with the connector provided) or pump tubing “drop to drop” to avoid introducing air into the column.
- Remove the snap-off end at the column outlet. Wash out the ethanol with at least 5 column volumes of distilled water or binding buffer.
- Equilibrate the column with at least 5 column volumes of binding buffer at 1 mL/min or 5 mL/min for 1 mL and 5 mL columns, respectively.
- Apply the sample using a syringe fitted to the Luer connector or by pumping it onto the column1.
- Wash with 5 to 10 column volumes of binding buffer or until no material appears in the effluent.
- Elute with 5 column volumes of elution buffer. The eluted fractions can be buffer exchanged using a prepacked desalting column (Chapter 11, Desalting/buffer exchange and concentration).
- After elution, regenerate the column by following the procedure described in Appendix 3 (Characteristics of Dextrin Sepharose High Performance products).
1 A lower flow rate (0.5 mL/min or 2.5 mL/min for 1 mL and 5 mL columns, respectively) can be used during sample application to optimize performance. The correlation between flow rate and number of drops is: a rate of 0.5mL/min corresponds to approximately 15 drops/min when using a syringe with a HiTrap 1 mL column, and 2.5 mL/min corresponds to approximately 60 drops/min when using a HiTrap 5 mL column.
Scaling up from 1 mL to 5 mL MBPTrap HP columns is easily performed by increasing sample load and flow rate five-fold. An alternative method for quick scale-up is to connect two or three MBPTrap HP columns in series (back pressure will increase). Figure 6.7 for an example of scale-up using this medium.
MBPTrap HP columns are easily cleaned with 0.5 M NaOH.
Store MBPTrap HP columns in 20% ethanol at 2 °C to 8 °C. After storage, equilibrate with binding buffer before use.
Application examples
1. Automated Two-step Purification on ÄKTAxpress
MBPTrap HP 1 mL was used as the first affinity step in an automated two-step purification on ÄKTAxpress. The second step, SEC, was run on HiLoad 16/60 Superdex 200 pg. MBP2*-paramyosin-δ-Sal (Mr ~70 000), which exists as a multimer in solution, was purified from E. coli lysate. Figure 6.3 shows the running conditions and the resulting chromatogram of the automated purification. Total final yield after the two steps was 2.16 mg, and the overall run time was only 3.4 h. The SDS-PAGE analysis in Figure 6.4 shows the high purity of the pooled fraction from the final SEC step.

Figure 6.3.Automated purification of MBP2*-paramyosin-δ-Sal using the two-step AC-SEC protocol on MBPTrap HP 1 mL (AC) and HiLoad 16/60 Superdex 200 pg (SEC).

Figure 6.4.SDS-PAGE analysis (reducing conditions) of the purification of MBP2*-paramyosin-δ-Sal.
2. Purification of a Protein Involved in Metabolic Disease
An MBPTrap HP column was used in a purification procedure for medium-chain acyl-CoA dehydrogenase (MCAD). This homotetramer (Mr 85 500), which is involved in metabolic disease, was purified for stability, folding, and kinetic studies (Figure 6.5). In addition to AC, the purification procedure also included an SEC step.
The purity of the eluted fractions from MBPTrap HP and SEC was determined by SDS-PAGE analysis. Some additional proteins besides the target protein were detected after the affinity step. This may be due to the presence of truncated variants still having the N-terminal MBP-tag intact, or possibly E. coli proteins associated with the target protein (this was not evaluated further). Final purity after SEC was extremely high according to SDS-PAGE analysis (Figure 6.6). Final yield was approximately 8.4 mg MCAD.

Figure 6.5.Purification of MCAD on (A) MBPTrap HP followed by (B) Superdex 200 pg.
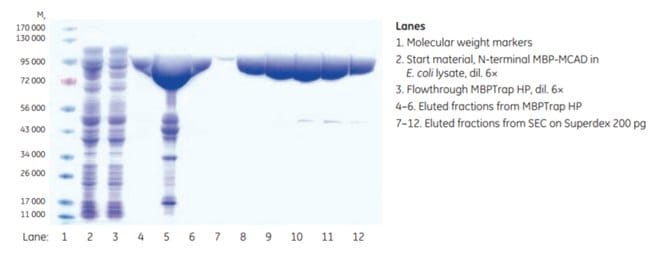
Figure 6.6.SDS-PAGE analysis (reducing conditions) of fractions from the two-step purification of MCAD. Data kindly provided by Dr. Esther M. Maier and Dr. von Haunersches Kinderspital, Munich, Germany.
3. Scaling Up
Scale-up can be achieved by increasing the bed volume while keeping the residence time constant. This approach maintains chromatographic performance during scale-up. MBP2*-β-galactosidase (Mr ~158 000), an affinity-tagged multimer, was purified on an MBPTrap HP 1 mL column on an ÄKTA chromatography system. The purification was scaled up on an MBPTrap HP 5 mL and an XK 26/20 column packed with Dextrin Sepharose® High Performance. The protein load was increased five-fold in each step (~10, ~50, and ~250 mg, respectively) and the residence time was ~2 min for all three columns. Figure 6.7 shows running conditions for all runs and the chromatograms from the MBPTrap HP 1 mL and Dextrin Sepharose® High Performance XK 26/20 runs. Figure 6.8 shows the SDS-PAGE results. The columns gave comparable results with high purity and similar yields (approximately 60%, Table 6.1), confirming the ease and reproducibility of scaling up purifications from MBPTrap HP columns to an XK 26/20 column. An alternative method for quick scale-up is to connect two or three MBPTrap HP columns in series, but this may increase back pressure.
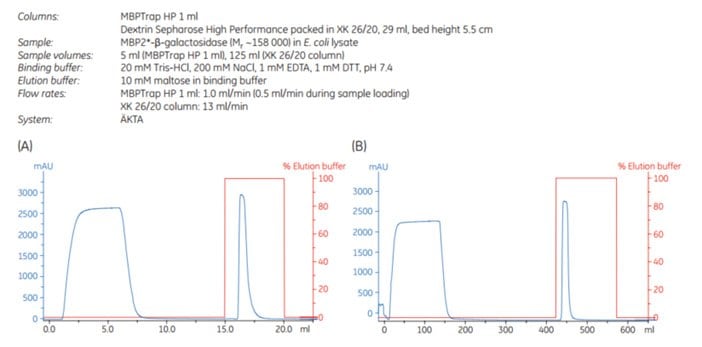
Figure 6.7.Scale-up of MBP2*-β-galactosidase purification, (A) MBPTrap HP 1 mL (B) Dextrin Sepharose® High Performance XK 26/20, 29 mL. Chromatogram for MBPTrap 5 mL column not shown.
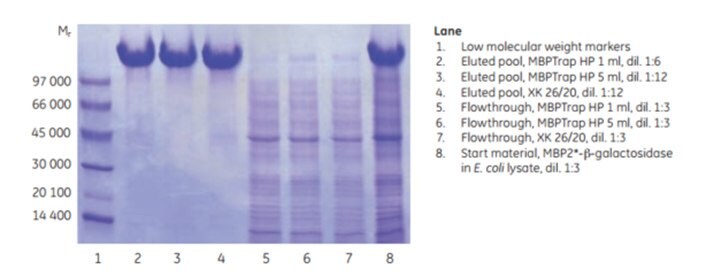
Figure 6.8.SDS-PAGE analysis (reducing conditions) of the scale-up study.
如要继续阅读,请登录或创建帐户。
暂无帐户?