358835
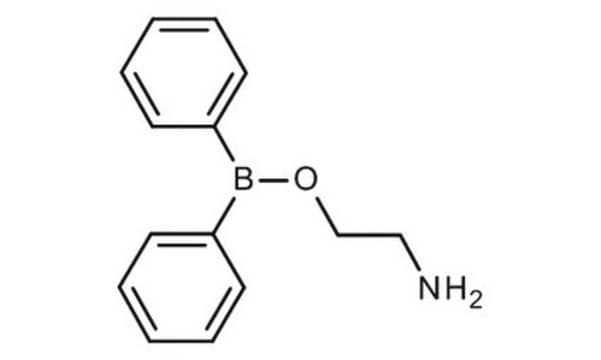
Diphenylborinic anhydride
95%
Synonym(s):
Oxybis(diphenylborane), Tetraphenyldiboroxane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C6H5)2BOB(C6H5)2
CAS Number:
Molecular Weight:
346.04
Beilstein:
2950331
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Assay
95%
form
solid
mp
135-140 °C (lit.)
fluorescence
λex 366 nm; λem 475 nm (after derivatization)
storage temp.
2-8°C
SMILES string
O(B(c1ccccc1)c2ccccc2)B(c3ccccc3)c4ccccc4
InChI
1S/C24H20B2O/c1-5-13-21(14-6-1)25(22-15-7-2-8-16-22)27-26(23-17-9-3-10-18-23)24-19-11-4-12-20-24/h1-20H
InChI key
SNQFEECGHGUHBK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Diphenylborinic anhydride (DPBA) may be used for the separation and determination of α-amino acids by boroxazolidone formation. DPBA, an analogue of the vascular gap junction channel blocker, 2-aminoethoxydiphenyl borate (2-APB), may be use in gap junctions channel studies.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ke-Tao Ma et al.
American journal of physiology. Heart and circulatory physiology, 300(1), H335-H346 (2010-11-03)
2-Aminoethoxydiphenyl borate (2-APB) analogs are potentially better vascular gap junction blockers than others widely used, but they remain to be characterized. Using whole cell and intracellular recording techniques, we studied the actions of 2-APB and its potent analog diphenylborinic anhydride
C J Strang et al.
Analytical biochemistry, 178(2), 276-286 (1989-05-01)
Reaction of an alpha-amino acid (alpha-AA) with 1,1-diphenylborinic acid (DPBA) leads to the formation of a kinetically stable adduct at pH 2-5 in which both the alpha-amino and the alpha-carboxyl groups are bound to boron forming a cyclic mixed anhydride
Yan Yang et al.
Biological & pharmaceutical bulletin, 34(9), 1390-1397 (2011-09-02)
2-Aminoethoxydiphenyl borate (2-APB) has recently been demonstrated to inhibit gap junction (GJ) channels, whereas the underlying mechanisms are still unknown. Using mouse TM₄ Sertoli cell which expresses connexin43 (Cx43), we explored the effects of 2-APB and its analogues on dye-coupling
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service