All Photos(1)
About This Item
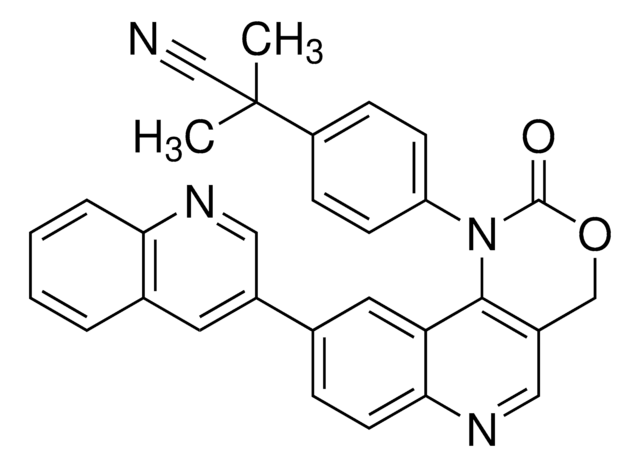
Empirical Formula (Hill Notation):
C20H29N3O2
CAS Number:
Molecular Weight:
343.46
EC Number:
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
Recommended Products
grade
analytical standard
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
cleaning products
cosmetics
food and beverages
forensics and toxicology
personal care
pharmaceutical (small molecule)
veterinary
format
neat
SMILES string
CCCCOc1cc(C(=O)NCCN(CC)CC)c2ccccc2n1
Looking for similar products? Visit Product Comparison Guide
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Biochem/physiol Actions
Potent, long-acting local anesthetic
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Seelig
Cell biology international reports, 14(4), 369-380 (1990-04-01)
The interaction between lipids and water soluble amphiphiles was investigated by means of a monolayer technique, monitoring the area increase at constant surface pressure. The area increase could be quantitated and binding isotherms at different surface pressures were measured. A
M Laura Parnas et al.
American journal of clinical pathology, 135(2), 271-276 (2011-01-14)
Butyrylcholinesterase (BChE) metabolizes the paralytic succinylcholine. Extended paralysis occurs in people with inherited BChE variants that may be identified by measuring BChE activity with and without the inhibitor dibucaine to calculate a dibucaine number (DN). Accurate phenotyping requires phenotype-specific BChE
Jamie Nelsen et al.
Pediatric emergency care, 25(10), 670-673 (2009-10-17)
Dibucaine is considered one of the most potent and consequently toxic amide anesthetics available, and despite withdrawal from the US market as a spinal anesthetic, it remains accessible as an over-the-counter preparation in the United States. Dibucaine exposures in children
Thaïs Souto-Padrón et al.
Parasitology research, 99(4), 317-320 (2006-04-14)
Although local anesthetics (LA) are considered primarily Na+-channel blockers in the past decade, an alternative action of LA as inhibitors of fusion among compartments of the endocytic/exocytic pathways was described. In epimastigote forms of Trypanosoma cruzi, we observed that 50
Zhicheng Wang et al.
Archives of biochemistry and biophysics, 495(2), 136-143 (2010-01-12)
There are evidence that both a disintegrin and metalloproteinase 17 (ADAM17) and calpain are involved in platelet glycoprotein (GP)Ibalpha ectodomain cleavage. However, the relationship between the two enzymes in the shedding process remains unclear. Here we show that calcium ionophore
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service