All Photos(1)
About This Item
Empirical Formula (Hill Notation):
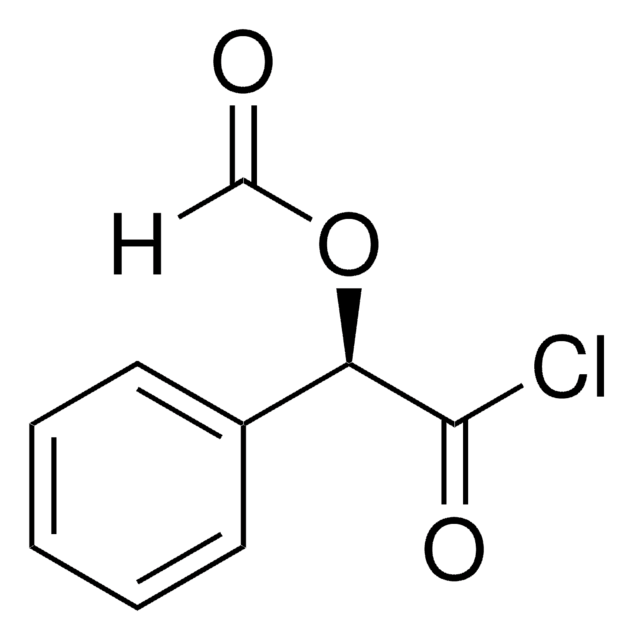
C4H8O2
Molecular Weight:
88.11
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
form
liquid
SMILES string
CC(O)CC=O
InChI
1S/C4H8O2/c1-4(6)2-3-5/h3-4,6H,2H2,1H3
InChI key
HSJKGGMUJITCBW-UHFFFAOYSA-N
Other Notes
Please note that Sigma-Aldrich provides this product to early discovery researchers as part of a collection of unique chemicals. Sigma-Aldrich does not collect analytical data for this product. Buyer assumes responsibility to confirm product identity and/or purity. All sales are final.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Dermal - Eye Irrit. 2
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amanda M Duran et al.
Protein science : a publication of the Protein Society, 27(1), 341-355 (2017-11-02)
Computational membrane protein design is challenging due to the small number of high-resolution structures available to elucidate the physical basis of membrane protein structure, multiple functionally important conformational states, and a limited number of high-throughput biophysical assays to monitor function.
B M Soares et al.
Physical chemistry chemical physics : PCCP, 19(2), 1181-1189 (2016-12-13)
A comprehensive study of the self-assembly in water of a lipopeptide consisting of a sequence of l-proline, l-arginine and l-tryptophan with a hydrocarbon chain has been performed. Fluorescence assays were used to determine the critical aggregation concentration. In situ small-angle
Haizea Echave et al.
Angewandte Chemie (International ed. in English), 55(10), 3364-3368 (2016-02-03)
The first enantioselective direct cross-aldol reaction of α-keto amides with aldehydes, mediated by a bifunctional ureidopeptide-based Brønsted base catalyst, is described. The appropriate combination of a tertiary amine base and an aminal, and urea hydrogen-bond donor groups in the catalyst
Asymmetric Catalysis Mediated by Synthetic Peptides, Version 2.0: Expansion of Scope and Mechanisms.
Anthony J Metrano et al.
Chemical reviews, 120(20), 11479-11615 (2020-09-25)
Low molecular weight synthetic peptides have been demonstrated to be effective catalysts for an increasingly wide array of asymmetric transformations. In many cases, these peptide-based catalysts have enabled novel multifunctional substrate activation modes and unprecedented selectivity manifolds. These features, along
Matthias Schapfl et al.
Applied microbiology and biotechnology, 102(19), 8359-8372 (2018-08-01)
Carboligations catalyzed by aldolases or thiamine diphosphate (ThDP)-dependent enzymes are well-known in biocatalysis to deliver enantioselective chain elongation reactions. A pyruvate-dependent aldolase (2-oxo-3-deoxy-6-phosphogluconate aldolase [EDA]) introduces a chiral center when reacting with the electrophile, glyoxylic acid, delivering the (S)-enantiomer of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service