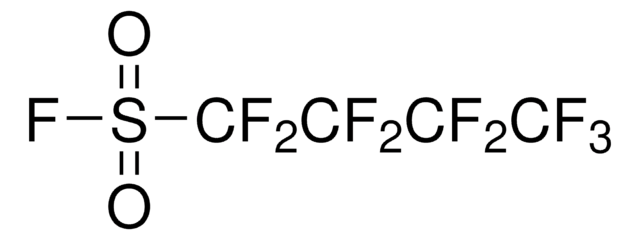804401
PyFluor
Synonym(s):
2-Pyridinesulfonyl Fluoride
About This Item
Recommended Products
Assay
95% (HPLC)
Quality Level
form
solid
mp
29-34 °C
storage temp.
2-8°C
SMILES string
O=S(C1=CC=CC=N1)(F)=O
InChI
1S/C5H4FNO2S/c6-10(8,9)5-3-1-2-4-7-5/h1-4H
InChI key
FCFXLXGZHDHJLB-UHFFFAOYSA-N
Related Categories
Application
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The prevalence of organofluorine compounds in industry and drug design necessitates the ability to introduce C–F bonds to molecules.
Related Content
Research in the Doyle group focuses on two areas: nucleophilic fluorination and nickel catalysis. The Doyle group has developed several reagents that advance these research areas. In fluorination, 2-pyridinesulfonyl fluoride (PyFluor) can be used for the mild deoxyfluorination of primary and secondary alcohols, a procedure which is normally accomplished by the sensitive reagent DAST. In nickel catalysis, the Doyle group has developed a modular, air-stable nickel precatalyst, [(TMEDA)Ni(o-tolyl)Cl], which has broad utility for a wide variety of reactions. This precatalyst can be used in place of Ni(cod)2, NiCl2, as well as other reported precatalysts. Doyle has also reported electron-deficient olefin ligands as a new class of ligand for accelerated reductive elimination. In particular, the sultam-derived ligand Fro-DO has been found to be critical for high yields in the cross-coupling of tertiary aziridines to form quaternary centers.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)