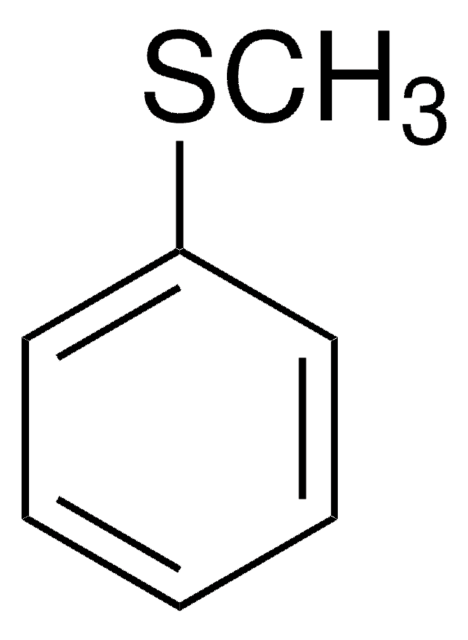
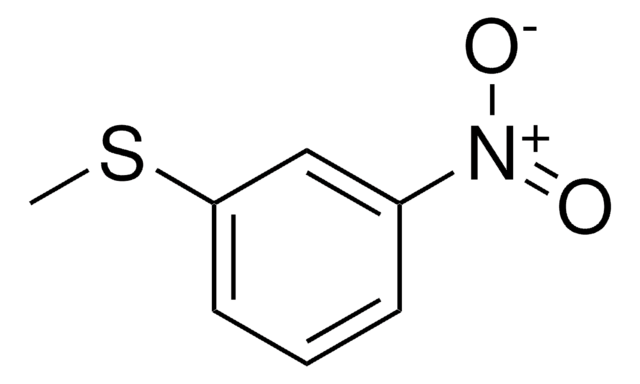
558001
2-Chlorothioanisole
96%
Synonym(s):
2-Chlorophenyl methyl sulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4SCH3
CAS Number:
Molecular Weight:
158.65
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
refractive index
n20/D 1.608 (lit.)
bp
239-240 °C (lit.)
SMILES string
CSc1ccccc1Cl
InChI
1S/C7H7ClS/c1-9-7-5-3-2-4-6(7)8/h2-5H,1H3
InChI key
IHLDFHCSSCVPQW-UHFFFAOYSA-N
General description
2-Chlorothioanisole can be prepared from the reaction between N,N-dimethylformamide, 1,2-dichlorobenzene, finely ground KOH and methyl mercaptan. Benzene dipole moment value analysis of 2-chlorothioanisole indicates that it exists predominantly in trans- form. 1H NMR spectrum of 2-chlorothioanisole has been studied in acetone-d6 solution.
Application
2-Chlorothioanisole may be used to synthesize 2-chlorophenyl methyl sulfone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
158.0 °F - closed cup
Flash Point(C)
70 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The preferred conformations of 2, 4-dichlorophenol, 2, 4-dichloroanisole and their sulphur analogues.
Lumbroso H, et al.
Journal of Molecular Structure, 43(1), 87-95 (1978)
Development of the Large-Scale Preparation of 2-(Methanesulfonyl) benzenesulfonyl Chloride.
Meckler H and Herr RJ.
Organic Process Research & Development, 16(4), 550-555 (2012)
Analysis of chlorophenylmethylsulfides in the urine of rats injected with chlorobenzene by high performance liquid chromatography.
M Yoshida et al.
Industrial health, 23(4), 283-287 (1985-01-01)
The proximate coupling constant, 5 J (H, CH3), and the torsional mobility of the thiomethyl group in some thioanisole derivatives.
Schaefer T, et al.
Canadian Journal of Chemistry, 69(4), 620-624 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service