All Photos(1)
About This Item
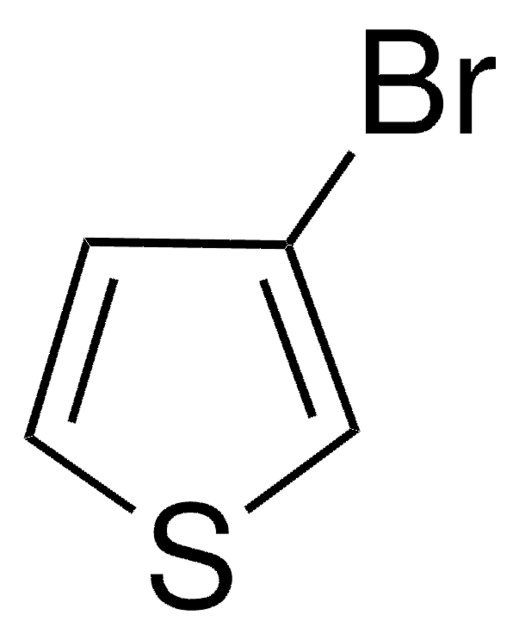
Linear Formula:
CH3C6H3(Br)I
CAS Number:
Molecular Weight:
296.93
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.65 (lit.)
bp
263 °C (lit.)
density
2.08 g/mL at 25 °C (lit.)
SMILES string
Cc1cc(Br)ccc1I
InChI
1S/C7H6BrI/c1-5-4-6(8)2-3-7(5)9/h2-4H,1H3
InChI key
GHTUADBHTFHMNI-UHFFFAOYSA-N
General description
5-Bromo-2-iodotoluene is a halogenated hydrocarbon. It undergoes chemoselective Suzuki reaction with phenylboronic acid to yield the corresponding carboxylic acid.
Application
5-Bromo-2-iodotoluene may be used in the synthesis of:
- 2-bromo-5-(4-bromo-2-methylphenyl)-3-methylpyridine
- 4-bromo-4′-(carbazol-9-yl)-2-methylbiphenyl
- 4,4′-dibromo-2-methyl-biphenyl
- 3-methyl-4-phenylbromobenzene
- 4-(4-bromo-2-methylphenyl)butanoic acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of comb polyphenylenes by Suzuki coupling from AB macromonomers.
Zhou S, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 52(11), 1519-1524 (2014)
Synthesis of new phenylpyridyl scaffolds using the Garlanding approach.
Voisin-Chiret AS, et al.
Tetrahedron, 66(40), 8000-8005 (2010)
Synthesis, Properties and Applications of Biphenyl Functionalized 9, 9-Bis (4-diphenylaminophenyl) fluorenes as Bifunctional Materials for Organic Electroluminescent Devices.
Thangthong A-M, et al.
European Journal of Organic Chemistry, 27, 5263-5274 (2012)
M Jonathan Fray et al.
Journal of medicinal chemistry, 46(16), 3514-3525 (2003-07-25)
The pathology of chronic dermal ulcers is characterized by excessive proteolytic activity which degrades extracellular matrix (required for cell migration) and growth factors and their receptors. The overexpression of MMP-3 (stromelysin-1) and MMP-13 (collagenase-3) is associated with nonhealing wounds, whereas
E Scott Priestley et al.
Journal of medicinal chemistry, 58(15), 6225-6236 (2015-07-08)
On the basis of a crystal structure of a phenylpyrrolidine lead and subsequent molecular modeling results, we designed and synthesized a novel series of macrocyclic FVIIa inhibitors. The optimal 16-membered macrocycle was 60-fold more potent than an acyclic analog. Further
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service