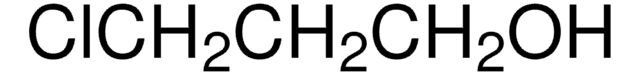All Photos(1)
About This Item
Linear Formula:
(CH3)3CSi(CH3)2O(CH2)4I
CAS Number:
Molecular Weight:
314.28
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
contains
copper as stabilizer
refractive index
n20/D 1.48 (lit.)
bp
75 °C/0.5 mmHg (lit.)
density
1.214 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)[Si](C)(C)OCCCCI
InChI
1S/C10H23IOSi/c1-10(2,3)13(4,5)12-9-7-6-8-11/h6-9H2,1-5H3
InChI key
INGJYKISFRSCQV-UHFFFAOYSA-N
General description
tert-Butyl(4-iodobutoxy)dimethylsilane is an iodoorganosilane that can be synthesized from tert-butyl(chloro)dimethylsilane.
Application
tert-Butyl(4-iodobutoxy)dimethylsilane may be used as a reagent in the multi-step synthesis of spermine synthon and decanucleotide-spermine conjugates.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fleming I.
Science of Synthesis: Houben-Weyl Methods of Molecular Transformations, 4, 266-266 (2014)
Online synthesis of diblock cationic oligonucleotides for enhanced hybridization to their complementary sequence.
Bénédicte Pons et al.
Chembiochem : a European journal of chemical biology, 7(8), 1173-1176 (2006-07-29)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service