All Photos(1)
About This Item
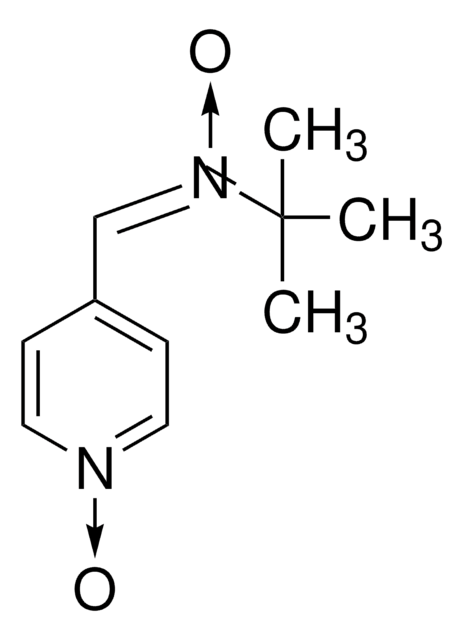
Empirical Formula (Hill Notation):
C8H15NO
CAS Number:
Molecular Weight:
141.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
bp
73 °C/1 mmHg (lit.)
mp
58-61 °C (lit.)
storage temp.
−20°C
SMILES string
CC1(C)CC(C)(C)[N+]([O-])=C1
InChI
1S/C8H15NO/c1-7(2)5-8(3,4)9(10)6-7/h6H,5H2,1-4H3
InChI key
GUQARRULARNYQZ-UHFFFAOYSA-N
General description
The ESR spectrum of 3,3,5,5-tetramethyl-1-pyrroline N-oxide was studied.
Application
3,3,5,5-Tetramethyl-1-pyrroline N-oxide was used as a spin traping reagent.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S Unchern et al.
Neurochemical research, 23(1), 97-102 (1998-03-03)
We compared neurotoxicity of piperine and low K+ on cultured cerebellar granule neurons. As considered from lactate dehydrogenase release and 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide reduction, both piperine and shifting from high K+ (25 mM) to low K+ (5.4 mM) were
G M Rosen et al.
Journal of medicinal chemistry, 31(2), 428-432 (1988-02-01)
Two nitrones, 3,3-diethyl-5,5-dimethylpyrroline 1-oxide (DEDMPO) and 3,3,5,5-tetramethylpyrroline 1-oxide (M4PO), were synthesized by the zinc/ammonium chloride reduction of appropiately substituted gamma-nitrocarbonyl compounds, followed by addition of methylmagnesium bromide to the resulting intermediate nitrones. The lipophilicities of these nitrones were estimated by
J F Torres-Roca et al.
Journal of immunology (Baltimore, Md. : 1950), 165(9), 4822-4830 (2000-10-25)
The roles of oxygen and reactive oxygen intermediates in apoptosis are unclear at present. Although oxygen and reactive oxygen intermediates are not required for the execution of apoptosis, oxygen may be involved in at least some forms of apoptosis. In
D De Bono et al.
Free radical research, 20(5), 327-332 (1994-05-01)
The hydroxyl radical adducts of 5,5 dimethyl-1-pyrolline-N-oxide (DMPO) and 3,3,5,5 tetramethyl-1-pyrolline-N-oxide (TMPO) formed in the presence of hydrogen peroxide and FeII are normally quite stable, but in the presence of 5-20 micromolar myoglobin their ESR signals decay rapidly. This decay
M J Davies et al.
The Biochemical journal, 240(3), 789-795 (1986-12-15)
Spin trapping using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) has been used to detect and distinguish between the carbon-centred, alkoxyl, and peroxyl radicals produced during the photolytic decomposition of hydroperoxides. Photolysis of tert-butyl and cumene hydroperoxides, and peroxidized fatty acids, in toluene, with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service