All Photos(2)
About This Item
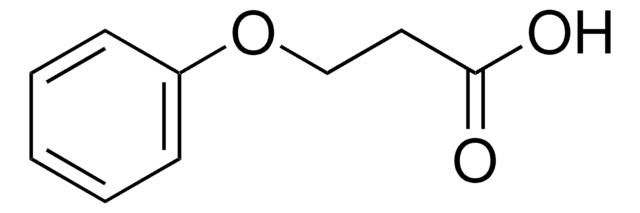
Linear Formula:
C6H5OCH(CH3)CO2H
CAS Number:
Molecular Weight:
166.17
Beilstein:
5734971
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
bp
265 °C (lit.)
mp
112-115 °C (lit.)
SMILES string
CC(Oc1ccccc1)C(O)=O
InChI
1S/C9H10O3/c1-7(9(10)11)12-8-5-3-2-4-6-8/h2-7H,1H3,(H,10,11)
InChI key
SXERGJJQSKIUIC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Solvent-induced invesrsion of enantioselectivity during esterification of 2-phenoxypropionic acid catalyzed by Candida cylindracea lipase has been investigated. Derivatives of 2-phenoxypropionic acid are potential herbicides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Manuel A P Segurado et al.
The Journal of organic chemistry, 72(14), 5327-5336 (2007-06-15)
Rate constants were measured for the oxidative chlorodehydrogenation of (R,S)-2-phenoxypropanoic acid and nine ortho-, ten para- and five meta-substituted derivatives using (R,S)-1-chloro-3-methyl-2,6-diphenylpiperidin-4-one (NCP) as chlorinating agent. The kinetics was run in 50% (v/v) aqueous acetic acid acidified with perchloric acid
Lipase-catalyzed production of optically active acids via asymmetric hydrolysis of esters.
Cambou B and Klibanov AM.
Applied Biochemistry and Biotechnology, 9(3), 255-260 (1984)
Solvent-induced inversion of enantiosflectivity in lipase-catalyzed esterification of 2-phenoxypropionic acids.
Ueji S, et al.
Biotechnology Letters, 14(3), 163-168 (1992)
Yan He et al.
Journal of pharmaceutical sciences, 95(1), 97-107 (2005-11-30)
The efficiency of a solubilization technique is determined by the physical-chemical properties of the drug. This study investigates the solubilization on two structurally related anticancer drugs, XK-469 and PPA. XK-469 is much less polar than PPA with an intrinsic solubility
Jessica Lin et al.
Journal of chromatography. A, 1624, 461244-461244 (2020-06-17)
Analysis and control of stereoisomers is a major task in pharmaceutical analysis, and is a greater challenge when compounds with multiple chiral centers (MCC) are concerned. HPLC and SFC are commonly used for stereoisomer analysis in drug development, typically starting
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service