81838
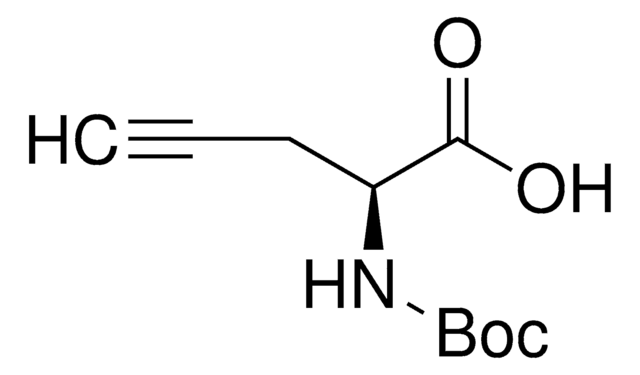
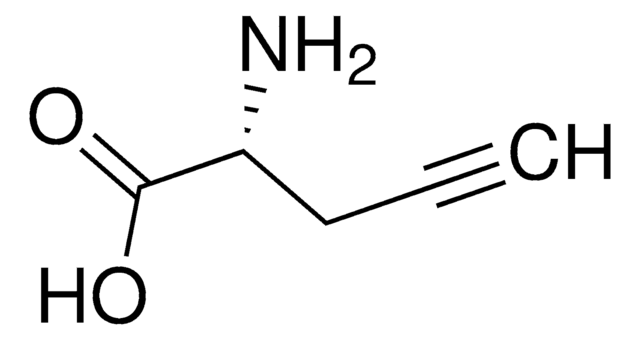
L-C-Propargylglycine
≥99.0% (TLC)
Synonym(s):
L-Propargylglycine, (S)-2-Amino-4-pentynoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H7NO2
CAS Number:
Molecular Weight:
113.11
Beilstein:
2347861
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
L-C-Propargylglycine, ≥99.0% (TLC)
Quality Level
Assay
≥99.0% (TLC)
form
powder
color
white
mp
235-239 °C
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
N[C@@H](CC#C)C(O)=O
InChI
1S/C5H7NO2/c1-2-3-4(6)5(7)8/h1,4H,3,6H2,(H,7,8)/t4-/m0/s1
InChI key
DGYHPLMPMRKMPD-BYPYZUCNSA-N
Application
Reagent for the irreversible inactivation of γ-cystathionase; affinity labeling reagent for γ-cystathionase and other enzymes; peptides containing this amino acid can be tritiated to high specific radioactivity
Biochem/physiol Actions
L-C-Propargylglycine, a specific inhibitor of H(2)S synthase of cystathionine-γ-lyase (CSE), may be used to study the role of H2S in regulation of biological processes.
L-propargylglycine (PAG), an inhibitor of cystathionine γ-lyase (CSE), is useful in studies of hydrogen sulphide synthesis and bioactivity.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Bridget Fox et al.
Journal of cellular and molecular medicine, 16(4), 896-910 (2011-06-18)
Hydrogen sulfide (H(2)S) has recently been proposed as an endogenous mediator of inflammation and is present in human synovial fluid. This study determined whether primary human articular chondrocytes (HACs) and mesenchymal progenitor cells (MPCs) could synthesize H(2)S in response to
Mechanism of inactivation of gamma-cystathionase by the acetylenic substrate analogue propargylglycine.
W Washtien et al.
Biochemistry, 16(11), 2485-2491 (1977-05-31)
Metabolic consequences of affinity labeling of cystathionase and alanine aminotransferase by L-propargylglycine in vivo.
S Shinozuka et al.
European journal of biochemistry, 124(2), 377-382 (1982-05-17)
Shiau Wei Lee et al.
Glia, 54(2), 116-124 (2006-05-24)
Hydrogen sulphide (H2S), which is produced endogenously from L-cysteine in mammalian tissues, has been suggested to function as a neuromodulator in the brain. However, the role of H2S in microglial cells is unclear. In this study, the effect of exogenous
A.N. Eberle et al.
Helvetica Chimica Acta, 68, 1880-1880 (1985)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service