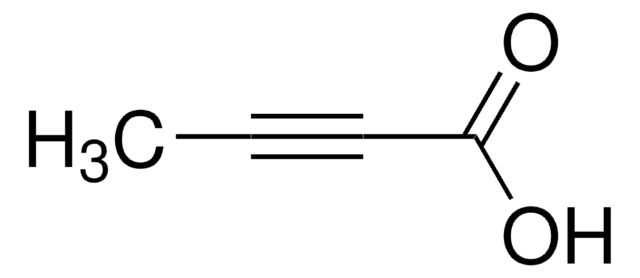P31205
Phenylpropiolic acid
99%
Synonym(s):
Phenylpropynoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5C≡CCOOH
CAS Number:
Molecular Weight:
146.14
Beilstein:
742587
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
mp
135-137 °C (lit.)
storage temp.
2-8°C
SMILES string
OC(=O)C#Cc1ccccc1
InChI
1S/C9H6O2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-5H,(H,10,11)
InChI key
XNERWVPQCYSMLC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Phenylpropiolic acid can:
- React with 2-tert-butoxypyridine in the presence of boron trifluoride·diethyl etherate to form the corresponding tert-butyl ester.
- Undergo decarboxylative coupling with aryl halides such as p-chloroiodobenzene and 1-chloro-4-iodobenzene.
- Undergo halodecarboxylation to form 1-haloalkynes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rocío Gómez-Vásquez et al.
Annals of botany, 94(1), 87-97 (2004-05-18)
Control of diseases in the key tropical staple, cassava, is dependent on resistant genotypes, but the innate mechanisms are unknown. The aim was to study phenylpropanoids and associated enzymes as possible defence components. Phenylalanine ammonia-lyase (PAL), phenylpropanoids and peroxidases (POD)
Neil R McIntyre et al.
Journal of enzyme inhibition and medicinal chemistry, 31(4), 551-562 (2015-05-30)
Peptidylglycine α-amidating monooxygenase (PAM) is a bifunctional enzyme that catalyzes the final reaction in the maturation of α-amidated peptide hormones. Peptidylglycine α-hydroxylating monooxygenase (PHM) is the PAM domain responsible for the copper-, ascorbate- and O2-dependent hydroxylation of a glycine-extended peptide.
M Zieliński et al.
Isotopes in environmental and health studies, 37(3), 239-252 (2002-04-02)
13C kinetic isotope effect (KIE) in the decarboxylation of phenylpropiolic acid (PPA) in tetralin medium (Tn) has been determined at 409-432 K and found to be of magnitude similar to the 13C KIE observed in the decarboxylation of malonic acid
Kun Li et al.
Ying yong sheng tai xue bao = The journal of applied ecology, 21(7), 1779-1784 (2010-10-01)
Taking the tissue-cultured seedlings of grape cultivar Red Globe as test objects, this paper examined the effects of their root aqueous extracts on seedling's growth, with the allelochemicals identified by LC-MS. The results showed that 0.02 g x ml(-1) (air-dried
A fast and practical synthesis of tert-butyl esters from 2-tert-butoxypyridine using boron trifluoride? diethyl etherate under mild conditions.
La M T and Kim H K
Tetrahedron, 74(27), 3748-3754 (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service