P5396
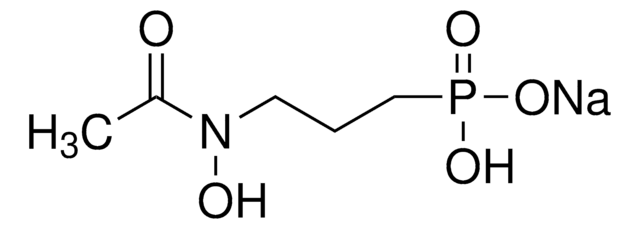
Phosphomycin disodium salt
antibacterial MurA inhibitor
Sinónimos:
(−)-(1R,2S)-(1,2-Epoxypropyl)phosphonic acid, Fosfomycin, Phosphonomycin
About This Item
Productos recomendados
Quality Level
form
powder
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mode of action
cell wall synthesis | interferes
storage temp.
2-8°C
SMILES string
[Na+].[Na+].C[C@@H]1O[C@@H]1P([O-])([O-])=O
InChI
1S/C3H7O4P.2Na/c1-2-3(7-2)8(4,5)6;;/h2-3H,1H3,(H2,4,5,6);;/q;2*+1/p-2/t2-,3+;;/m0../s1
InChI key
QZIQJIKUVJMTDG-JSTPYPERSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
Biochem/physiol Actions
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico