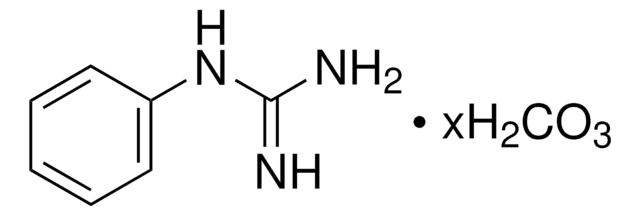
H7654
Hydroxyguanidine sulfate salt
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH5N3O · 1/2H2SO4
Número de CAS:
Peso molecular:
124.11
Número CE:
Número MDL:
Código UNSPSC:
12352202
ID de la sustancia en PubChem:
NACRES:
NA.77
Productos recomendados
origen biológico
synthetic (organic)
Nivel de calidad
Ensayo
≥98% (TLC)
Formulario
powder
solubilidad
water: 25 mg/mL, clear, colorless
temp. de almacenamiento
2-8°C
cadena SMILES
NC(=N)NO.NC(=N)NO.OS(O)(=O)=O
InChI
1S/2CH5N3O.H2O4S/c2*2-1(3)4-5;1-5(2,3)4/h2*5H,(H4,2,3,4);(H2,1,2,3,4)
Clave InChI
MTGDDPZRXSDPFH-UHFFFAOYSA-N
Acciones bioquímicas o fisiológicas
An early antitumor agent. Oxidation results in release of NO, and formation of other reactive oxygen species, including peroxynitrite and peroxyl radicals. Reacts with NO to form an adduct which is a potent and stable vasodilator.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Zhi-guo Zhang et al.
Yao xue xue bao = Acta pharmaceutica Sinica, 39(9), 705-710 (2004-12-21)
To search for novel antiasthmatic agents. Coupling seratrodast (SD), an antiasthmatic drug, with several different types of NO donors including oxatriazoles, N-hydroxyguanidines and furoxans; evaluating the antiasthmatic effects of coupled compounds by determining their inhibitory activity of guinea pig asthma
David Lefèvre-Groboillot et al.
The FEBS journal, 272(12), 3172-3183 (2005-06-16)
The binding of several alkyl- and aryl-guanidines and N-hydroxyguanidines to the oxygenase domain of inducible NO-synthase (iNOS(oxy)) was studied by UV/Vis difference spectroscopy. In a very general manner, monosubstituted guanidines exhibited affinities for iNOS(oxy) that were very close to those
Patrick Slama et al.
Biochemical and biophysical research communications, 316(4), 1081-1087 (2004-03-27)
Conversion of neurotransmitter dopamine into norepinephrine is catalyzed by dopamine beta-hydroxylase (DbH). The reaction requires the presence of both molecular oxygen and a reducing cosubstrate, the assumed physiological cosubstrate being ascorbic acid. We have investigated the ability of a new
Daniel Mansuy et al.
Free radical biology & medicine, 37(8), 1105-1121 (2004-09-29)
Nitric oxide (NO) is a key inter- and intracellular molecule involved in the maintenance of vascular tone, neuronal signaling, and host response to infection. The biosynthesis of NO in mammals involves a two-step oxidation of L-arginine (L-Arg) to citrulline and
Patrick Slama et al.
Journal of inorganic biochemistry, 103(3), 455-462 (2009-01-31)
N-Aryl-N'-hydroxyguanidines are compounds that display interesting pharmacological properties but their chemical reactivity remains poorly investigated. Some of these compounds are substrates for the heme-containing enzymes nitric-oxide synthases (NOS) and act as reducing co-substrates for the copper-containing enzyme Dopamine beta-Hydroxylase (DBH)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico