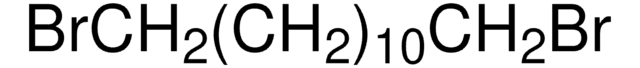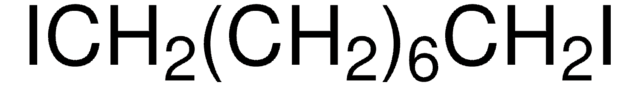D39800
1,10-Dibromodecane
97%
Sinónimos:
Decamethylene dibromide
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
Br(CH2)10Br
Número de CAS:
Peso molecular:
300.07
Beilstein/REAXYS Number:
506156
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
form
crystals
refractive index
n20/D 1.4912 (lit.)
bp
160 °C/15 mmHg (lit.)
mp
25-27 °C (lit.)
density
1.335 g/mL at 25 °C (lit.)
SMILES string
BrCCCCCCCCCCBr
InChI
1S/C10H20Br2/c11-9-7-5-3-1-2-4-6-8-10-12/h1-10H2
InChI key
GTQHJCOHNAFHRE-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
1,10-Dibromodecane, also known as Decamethylene dibromide, is an aromatic and aliphatic monomer, often utilized in the polymerization reaction to synthesize high molecular weight polysulfides through polycondensation.
Application
1,10-Dibromodecane is used:
- as a reactant in the Wurtz-type reaction during the synthesis of polyethylene
- Construction of pillar[4]arene[1]quinone-1,10-dibromodecane pseudorotaxanes in solution and in the solid state.: This research highlights the application of 1,10-Dibromodecane in constructing complex molecular structures known as pseudorotaxanes. The study focuses on the synthesis and stabilization of these structures both in solution and solid states, providing a foundational technique for developing advanced materials in chemical engineering, pharmaceutical synthesis, and high-performance materials within industrial chemical manufacturing sectors. This breakthrough offers potential pathways for new drug delivery systems and smart materials based on the unique properties of these supramolecular assemblies (Sheng et al., 2020).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
233.6 °F - closed cup
flash_point_c
112 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Stefania Aivali et al.
The journal of physical chemistry. B, 124(24), 5079-5090 (2020-05-28)
Conjugation-break flexible spacers in-between π-conjugated segments were utilized herein toward processable perylene diimide (PDI)-based polymers. Aromatic-aliphatic PDI-based polymers were developed via the two-phase polyetherification of a phenol-difunctional PDI monomer and aliphatic dibromides. These polyethers showed excellent solubility and film-forming ability
Ruili Zhang et al.
Advanced healthcare materials, 9(14), e2000394-e2000394 (2020-06-17)
The complexity of biological systems poses a great challenge in the development of nanotheranostic agents with enhanced therapeutic efficacies. To systematically overcome a series of barriers during in vivo administration and achieve optimal antitumor activity, nanotheranostic agents that can self-adaptively
Synthesis of polysulfides containing s-triazine rings from 6-substituted amino-1, 3, 5-triazine-2, 4-dithiols and 1, 10-dibromodecane
Yoshiyuki O, et al.
Macromolecular Rapid Communications, 20, 294-298 (1999)
Chemical structures, properties, and applications of selected crude oil-based and bio-based polymers
Piotr K, et al.
Polymers, 14, 5551-5551 (2022)
Design and Synthesis of Imidazolium-Mediated Tro?ger?s Base-Containing Ionene Polymers for Advanced CO2 Separation Membranes
Irshad k, et al.
ACS Omega, 4, 3439-3448 (2019)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico