902586
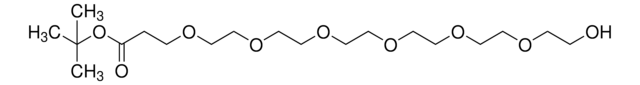
Hydroxy-PEG4-t-butyl ester
Sinónimos:
tert-Butyl-1-hydroxy-3,6,9,12-tetraoxapentadecan-15-oate, HO-PEG4-CO-OtBu
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C15H30O7
Número de CAS:
Peso molecular:
322.39
Número MDL:
Código UNSPSC:
51171641
NACRES:
NA.22
Productos recomendados
Ensayo
≥95%
Formulario
liquid
idoneidad de la reacción
reagent type: cross-linking reagent
índice de refracción
n/D 1.4492
densidad
1.04746 g/mL
grupo funcional
ester
hydroxyl
temp. de almacenamiento
2-8°C
cadena SMILES
O=C(OC(C)(C)C)CCOCCOCCOCCOCCO
InChI
1S/C15H30O7/c1-15(2,3)22-14(17)4-6-18-8-10-20-12-13-21-11-9-19-7-5-16/h16H,4-13H2,1-3H3
Clave InChI
FJRDXEGYAVAMLB-UHFFFAOYSA-N
Categorías relacionadas
Aplicación
This heterobifunctional, PEGylated crosslinker features a hydroxyl group at one end and t-butyl-protected carboxylic acid at the other, which can be deprotected with acidic conditions. The hydrophillic PEG linker facilitates solubility in biological applications. Hydroxy-PEG4-t-butyl ester can be used for bioconjugation or as a building block for synthesis of small molecules, conjugates of small molecules and/or biomolecules, or other tool compounds for chemical biology and medicinal chemistry that require ligation. Examples of applications include its synthetic incorporation into antibody-drug conjugates or proteolysis-targeting chimeras (PROTAC® molecules) for targeted protein degradation.
Otras notas
Información legal
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
Producto relacionado
Referencia del producto
Descripción
Precios
Código de clase de almacenamiento
10 - Combustible liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Proline-functionalized magnetic core-shell nanoparticles as efficient and recyclable organocatalysts for aldol reactions.
Yacob Z, et al
Advanced Synthesis & Catalysis, 354(17), 3259-3264 (2012)
A new route for the synthesis of 1-amino-3,6,9,12-?tetraoxapentadecan-15-oic acid.
Wu X, et al.
J. Chem. Res. (M), 40(6), 368-370 (2016)
Recruiting cytotoxic T cells to folate-receptor-positive cancer cells.
Sumith A Kularatne et al.
Angewandte Chemie (International ed. in English), 52(46), 12101-12104 (2014-02-28)
Venkata R Doppalapudi et al.
Bioorganic & medicinal chemistry letters, 17(2), 501-506 (2006-10-24)
Aryl sulfonamide-based endothelin antagonists were synthesized and covalently linked to the reactive lysine of the m38C2 antibody to create a series of CovX-Bodies. These chemically programmed antibodies behaved as potent endothelin receptor antagonists in vitro and had antitumor efficacy in
Sara V Orski et al.
Journal of the American Chemical Society, 132(32), 11024-11026 (2010-08-12)
Surfaces containing reactive ester polymer brushes were functionalized with cyclopropenone-masked dibenzocyclooctynes for the light activated immobilization of azides using catalyst-free click chemistry. The photodecarbonylation reaction in the amorphous brush layer is first order for the first 45 s with a
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico