763284
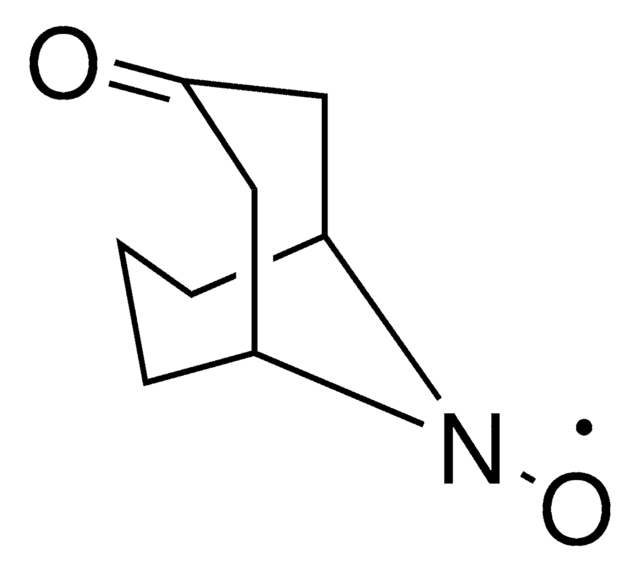
9-Azabicyclo[3.3.1]nonane N-oxyl
95%
Sinónimos:
9-Azabicyclo[3.3.1]nonane N-oxyl radical, ABNO
About This Item
Productos recomendados
assay
95%
form
solid
reaction suitability
reagent type: ligand
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
65-70 °C
greener alternative category
, Aligned
Storage temp.
2-8°C
SMILES string
C[C@@]12CCC[C@@](C)(CCC1)N2[O]
InChI
1S/C10H18NO/c1-9-5-3-7-10(2,11(9)12)8-4-6-9/h3-8H2,1-2H3/t9-,10+
Inchi Key
GGWCZKZSILYISB-AOOOYVTPSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
Copper(I)/ABNO-Catalyzed Aerobic Alcohol Oxidation: Alleviating Steric and Electronic Constraints of Cu/TEMPO Catalyst Systems
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Contenido relacionado
he Stahl Lab focuses on the development of catalysts and catalytic reactions for selective oxidation of organic molecules, with particular emphasis on aerobic oxidation reactions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico