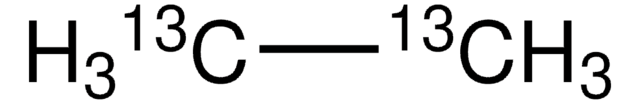539775
Ethane
99.99%
Sinónimos:
Ethane gas
About This Item
Productos recomendados
vapor density
1.05 (vs air)
Quality Level
vapor pressure
37.95 atm ( 21.1 °C)
assay
99.99%
form
gas
autoignition temp.
881 °F
expl. lim.
13 %
bp
−88 °C (lit.)
mp
−172 °C (lit.)
density
0.362 g/mL at 20 °C (lit.)
SMILES string
CC
InChI
1S/C2H6/c1-2/h1-2H3
InChI key
OTMSDBZUPAUEDD-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- High-pressure oxidation of ethane: The paper discusses the oxidation properties of ethane under high pressure, providing insights crucial for developing combustion models and understanding ethane′s behavior in various industrial processes (H Hashemi, JG Jacobsen, CT Rasmussen, 2017).
- Progress and prospects in catalytic ethane aromatization: This review highlights the advancements in converting ethane to more valuable aromatic hydrocarbons, showcasing the potential of ethane as a petrochemical feedstock (Y Xiang, H Wang, J Cheng, J Matsubu, 2018).
Packaging
Compatible with the following:
- Aldrich® lecture-bottle station systems
- Aldrich® lecture-bottle gas regulators
Other Notes
Legal Information
Optional
also commonly purchased with this product
control valve
hose barb
purge valve
regulator
signalword
Danger
hcodes
Hazard Classifications
Flam. Gas 1A - Press. Gas Liquefied gas
Storage Class
2A - Gases
wgk_germany
nwg
flash_point_f
-211.0 °F - closed cup
flash_point_c
-135 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Protocolos
Separation of Methane; Acetylene; Carbon monoxide; Water; Nitrogen; Carbon dioxide; Ethane; Ethylene
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico