304484
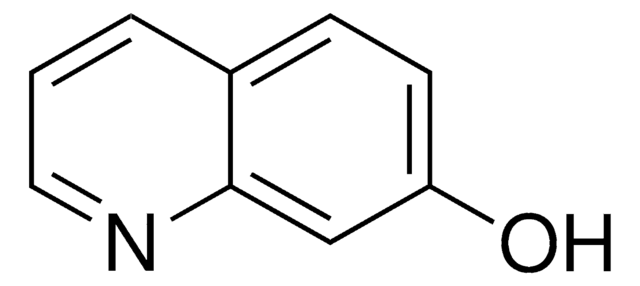
6-Hydroxyquinoline
95%
Sinónimos:
6-Quinolinol, 6-Hydroxyquinoline
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H7NO
Número de CAS:
Peso molecular:
145.16
Beilstein:
113196
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
95%
mp
188-190 °C (lit.)
cadena SMILES
Oc1ccc2ncccc2c1
InChI
1S/C9H7NO/c11-8-3-4-9-7(6-8)2-1-5-10-9/h1-6,11H
Clave InChI
OVYWMEWYEJLIER-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
6-Hydroxyquinoline is an ideal photoacid system for exploring excited-state proton transfer (ESPT) reactions. The excited-state proton transfer and geminate recombination of 6-hydroxyquinoline encaged in catalytic Na+-exchanged faujasite zeolites X and Y have been explored by measuring steady-state and picosecond time-resolved spectra.
Aplicación
6-Hydroxyquinoline was used in synthesis of 2,6-substituted-benzo[d]thiazole analogs and 2,4-substituted-benzo[d]thiazole analogs.
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Katharigatta Narayanaswamy Venugopala et al.
European journal of medicinal chemistry, 65, 295-303 (2013-06-04)
A novel and efficient one pot synthesis was developed for 2,6-substituted-benzo[d]thiazole analogues 4a-k and 2,4-substituted-benzo[d]thiazole analogues 4l-pvia three component condensation reaction of substituted arylaldehyde, 2-amino-6-halo/4-methyl-benzo[d]thiazole and 2-naphthol or 6-hydroxyquinoline in presence of 10% w/v NaCl in water by microwave method.
Yu-Hui Liu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 128, 280-284 (2014-04-01)
6-Hydroxyquinoline (6HQ) is an ideal photoacid system for exploring excited-state proton transfer (ESPT) reactions. We have previously (Mahata et al. (2002)) shown that the ESPT reaction between 6HQ and trimethylamine (TMA) leads to an "unusual" emission in the 440-450 nm
G Bott et al.
Biological chemistry Hoppe-Seyler, 372(6), 381-383 (1991-06-01)
Two strains, using 6-hydroxyquinoline as sole source of energy, carbon and nitrogen, have been isolated. These bacteria, designated 31/1 Fa1 and 31/2 A1, are also able to degrade quinoline. According to their physiological properties strain 31/1 Fa1 has been identified
Selective phenolic acylation of 10-hydroxycamptothecin using poly (ethylene glycol) carboxylic acid.
Richard B Greenwald et al.
Bioorganic & medicinal chemistry letters, 13(3), 577-580 (2003-02-05)
Selective acylation of the phenolic hydroxyl group of 10-hydroxycamptothecin has been accomplished using phenyl dichlorophosphate. Additional modification of the 10-OH as an ether permits a 20-acyl derivative to be synthesized. This result along with data from a 6-hydroxyquinoline model strongly
Anna Michta et al.
Acta crystallographica. Section C, Crystal structure communications, 65(Pt 2), o66-o69 (2009-02-05)
The title compound, C(9)H(7)NO, has two symmetry-independent molecules in the asymmetric unit, which have different conformations of the hydroxy group with respect to the quinoline ring. One of the molecules adopts a cis conformation, while the other shows a trans
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico