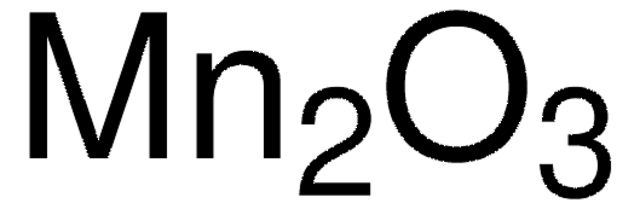215880
Manganese(III) acetate dihydrate
97%
Sinónimos:
Manganese triacetate dihydrate
About This Item
Productos recomendados
Quality Level
assay
97%
form
powder or chunks
reaction suitability
core: manganese
SMILES string
O.O.CC(=O)O[Mn](OC(C)=O)OC(C)=O
InChI
1S/3C2H4O2.Mn.2H2O/c3*1-2(3)4;;;/h3*1H3,(H,3,4);;2*1H2/q;;;+3;;/p-3
InChI key
ONJSLAKTVIZUQS-UHFFFAOYSA-K
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- A precursor in the synthesis of manganese oxide (Mn3O4) nanostructures, which are employed as anode materials in lithium-ion batteries.
- A manganese source in the sol-gel synthesis of Mn-doped ZnO thin films. The incorporation of manganese ions into the ZnO lattice is essential for modifying the electronic and optical properties of the films.
- A precursor for the synthesis of manganese oxide nanoparticles using a sol-gel process. These nanoparticles are evaluated for their performance in supercapacitor applications.
- A mild and selective oxidizing agent. Catalyzes allylic oxidation of a variety of alkenes in the presence of tert-butylhydroperoxide. Reagent used for radical cyclizations and α-keto-acetoxylation.
Physical form
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis, and are some of the organic chemist’s most powerful tools for creating novel products. Below is a list of the most commonly used oxidizing and reducing agents currently available in our catalog.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico