To target a carboxyl group on cellular proteins, the carbodiimide group of EDC must be unbound so the carbonyl from the carboxylic acid can initiate an attack. This interaction results in the formation of an O-acylisourea intermediate that can react with amines, with an urea by-product being released as a part of the process. Should a fluorophore be pre-conjugated to EDC, it is likely to be released as this by-product and thus would not attach to the carboxyl groups of the cells.
165344
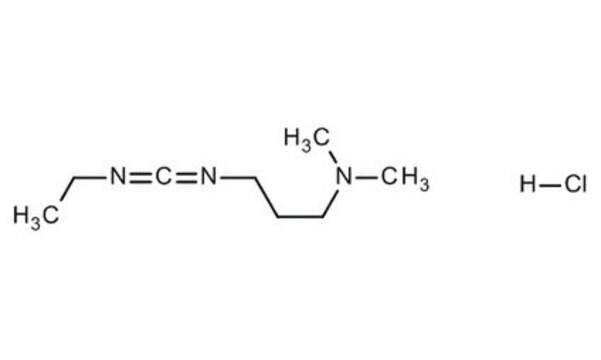
1-[3-(Dimethylamino)propyl]-3-ethylcarbodiimide methiodide
for peptide synthesis
Sinónimos:
EDC methiodide
About This Item
Productos recomendados
Nombre del producto
1-[3-(Dimethylamino)propyl]-3-ethylcarbodiimide methiodide,
Formulario
powder
Nivel de calidad
idoneidad de la reacción
reaction type: Coupling Reactions
mp
97-99 °C (lit.)
aplicaciones
peptide synthesis
grupo funcional
amine
temp. de almacenamiento
2-8°C
cadena SMILES
[I-].CCN=C=NCCC[N+](C)(C)C
InChI
1S/C9H20N3.HI/c1-5-10-9-11-7-6-8-12(2,3)4;/h5-8H2,1-4H3;1H/q+1;/p-1
Clave InChI
AGSKWMRPXWHSPF-UHFFFAOYSA-M
Categorías relacionadas
Aplicación
- Crosslinking agent
Reactant for:
- Amide bond forming (amidation) crosslinking reactions
Palabra de señalización
Warning
Frases de peligro
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
-
Is it possible to have EDC conjugated with a fluorophore such that it can still target Carboxyl groups on the cellular proteins
1 answer-
Helpful?
-
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico