155071
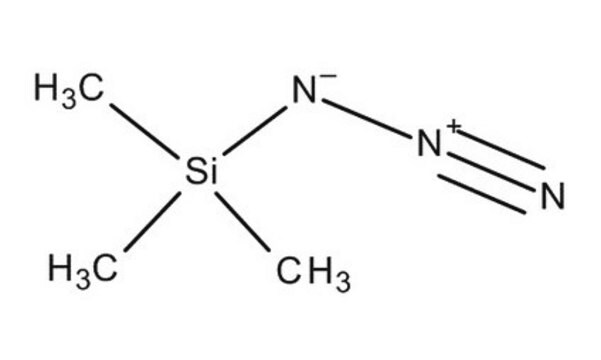
Azidotrimethylsilane
95%
Sinónimos:
Trimethylsilyl azide
About This Item
Productos recomendados
assay
95%
form
liquid
refractive index
n20/D 1.414 (lit.)
bp
52-53 °C/175 mmHg (lit.)
density
0.868 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C[Si](C)(C)N=[N+]=[N-]
InChI
1S/C3H9N3Si/c1-7(2,3)6-5-4/h1-3H3
InChI key
SEDZOYHHAIAQIW-UHFFFAOYSA-N
General description
Application
- A nitrogen precursor to prepare GaN nanowire via metal-organic chemical vapor deposition method.
- An electrolyte additive in Li-O2 batteries. The addition of TMSN3 results in the formation of robust solid electrolyte interphase.
- An efficient reagent in the synthesis of tetrazoles, fullerenyl azide, and α-azido oximes.
- A silylating agent in the O-trimethyl silylation of alcohols and phenols.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
42.8 °F - closed cup
flash_point_c
6 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico