General description
Collagen type I contributes maximum to the fibrous protein content in mammals. It is also present in arteries and extracellular matrix. The protein has an elongated cylindrical structure with tapering ends. It possess a left-handed helix with three polyproline II-type polypeptide strands.
Type I collagen [1α(I)]2α2 is distributed throughout the body. This fibrillar collagen is found in dermis, bone, tendon, ligament, dentin, fasciae, sclera, cornea, organ capsules and fibrous cartilage. It appears in tissues as the classically designated collagen fibers which are formed from densely-packed thin striated fibrils with marked variation in diameter. Collagen I is synthesized mainly by fibroblasts, osteoblasts, odontoblasts and chondroblasts.Collagen type Iα1 (COL1A1) is encoded by the gene mapped to human chromosome 17q21.33. It is the most abundant extracellular matrix (ECM) protein in humans. Type 1 collagen is the major structural protein of bone, tendon, skin and cornea. The encoded protein is a heterotrimer consisting of two α1-chains and one α2-chain.
Specificity
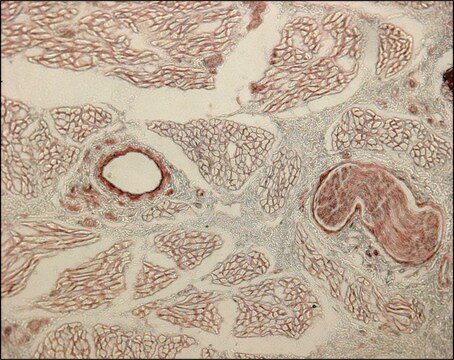
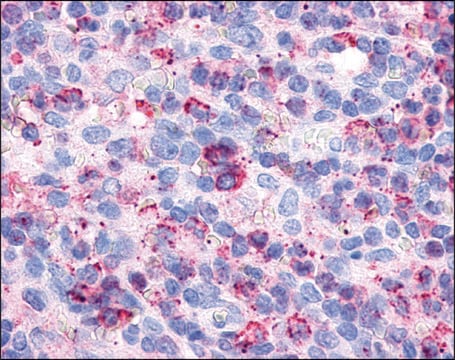
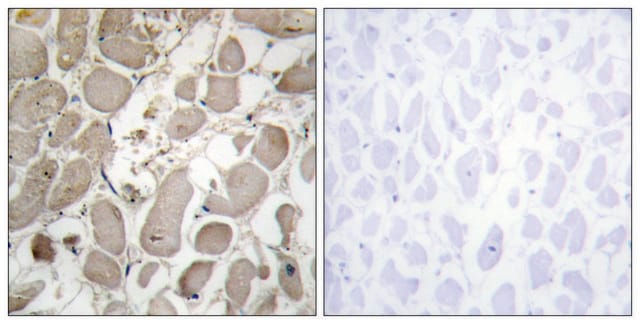
The antibody is reactive with the native (helical) form of collagen type I in ELISA and dot blot. The product is not reactive when tested on thermally denatured molecules. In immunohistochemical staining of acetone-fixed and unfixed frozen sections, a strong staining of connective tissue fibers is seen. Using the dot blot and ELISA techniques, the antibody shows no cross-reactivity with collagen types, II, III, IV, V, VI, VII, IX, X and XI. The epitope recognized by the antibody is sensitive to routine formalin fixation and paraffin embedding.
Immunogen
Bovine skin collagen type I
Application
Monoclonal Anti-Collagen Type I may be used for the localization of type I collagen using various immunochemical assays including ELISA, dot blot and immunohistochemistry. A minimum working dilution of 1:2,000 is determined by indirect immunofluorescent staining of human or other mammalian frozen sections.
Monoclonal Anti-Collagen, Type I antibody produced in mouse has been used in:
- immunohistochemistry
- dot blot technique
- indirect immunofluorescence staining
Biochem/physiol Actions
Collagen type I is responsible for mechanical stability, elasticity, strength and toughness, observed in tendons, ligaments, skin, cornea, bone, dentin and many other tissues.
Type 1 collagen serves a structural role in the extracellular matrix by providing mechanical support and resistance to tension. Some of the more important genetic diseases, directly or indirectly involving this collagen type include the majority cases of osteogenesis imperfecta and certain types of Ehlers Danlos syndrome. The development of antibodies against collagens has provided a powerful method for examining the distribution of these connective tissue proteins and for investigation of epithelial-mesenchymal interactions, tumorigenesis and basement membrane biology in ontogeny and epithelial differentiation.
Storage and Stability
Store at 2-8 °C for up to one month. For extended storage freeze in working aliquots. Repeated freezing and thawing is not recommended. Storage in "frost-free" freezers is not recommended. If slight turbidity occurs upon prolonged storage, clarify the solution by centrifugation before use.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.