13752
Penicillin G sodium salt
96.0-102.0%
Synonym(s):
Benzylpenicillin sodium salt
About This Item
Recommended Products
Quality Level
Assay
96.0-102.0%
form
powder
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
Mode of action
cell wall synthesis | interferes
storage temp.
2-8°C
SMILES string
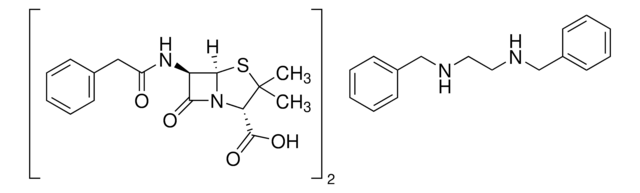
[Na+].[H][C@]12SC(C)(C)[C@@H](N1C(=O)[C@H]2NC(=O)Cc3ccccc3)C([O-])=O
InChI
1S/C16H18N2O4S.Na/c1-16(2)12(15(21)22)18-13(20)11(14(18)23-16)17-10(19)8-9-6-4-3-5-7-9;/h3-7,11-12,14H,8H2,1-2H3,(H,17,19)(H,21,22);/q;+1/p-1/t11-,12+,14-;/m1./s1
InChI key
FCPVYOBCFFNJFS-LQDWTQKMSA-M
Looking for similar products? Visit Product Comparison Guide
1 of 4
This Item | BR782153 | BR782152 | BR782151 |
|---|---|---|---|
| packaging pack of 100 ea | packaging pack of 50 ea | packaging pack of 50 ea | packaging pack of 100 ea |
| manufacturer/tradename BRAND 782150 | manufacturer/tradename BRAND 782153 | manufacturer/tradename BRAND 782152 | manufacturer/tradename BRAND 782151 |
| plate size 96 wells | plate size 1536 wells | plate size - | plate size 96 wells |
General description
Application
Biochem/physiol Actions
Antimicrobial spectrum: This product is active against gram-positive and gram-negative bacteria.
Packaging
Caution
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
β-lactam antibacterials inhibit transpeptidase enzymes, preventing peptidoglycan assembly in both Gram-positive and Gram-negative bacteria.
β-lactam antibacterials inhibit transpeptidase enzymes, preventing peptidoglycan assembly in both Gram-positive and Gram-negative bacteria.
β-lactam antibacterials inhibit transpeptidase enzymes, preventing peptidoglycan assembly in both Gram-positive and Gram-negative bacteria.
β-lactam antibacterials inhibit transpeptidase enzymes, preventing peptidoglycan assembly in both Gram-positive and Gram-negative bacteria.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service