69400
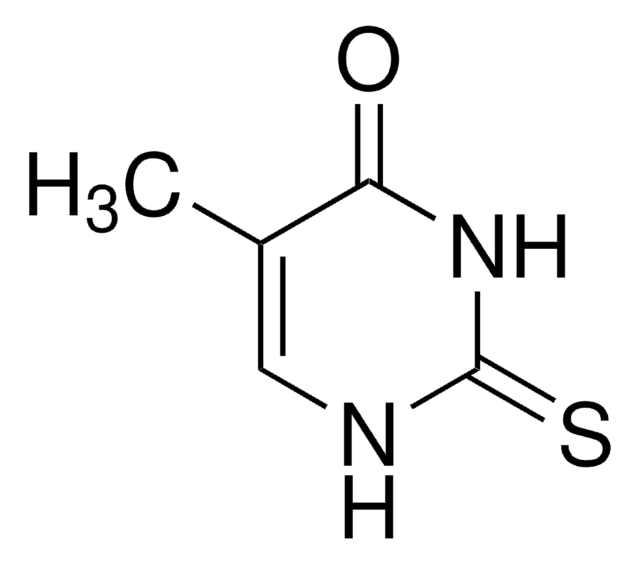
6-Methyl-2-thiouracil
purum, ≥98.0% S basis (elemental analysis)
Synonym(s):
Basethyrin, Methiocil, Thiothymin, 4-Hydroxy-2-mercapto 6-methylpyrimidine, MZU
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H6N2OS
CAS Number:
Molecular Weight:
142.18
Beilstein:
115648
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% S basis (elemental analysis)
form
crystals
mp
~330 °C (dec.) (lit.)
SMILES string
CC1=CC(=O)NC(=S)N1
InChI
1S/C5H6N2OS/c1-3-2-4(8)7-5(9)6-3/h2H,1H3,(H2,6,7,8,9)
InChI key
HWGBHCRJGXAGEU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
6-Methyl-2-thiouracil possesses antithyroid activity.
Application
6-Methyl-2-thiouracil can be used in:
- Synthesis of luminescent gold(I) thiouracilate complexes as emissive materials.
- Synthesis of uracil-containing histone deacetylase inhibitors.
- Synthesis of S-dihydro-alkylthio-benzyl-oxopyrimidines (S-DABOs) based anti-HIV agents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and biological properties of novel, uracil-containing histone deacetylase inhibitors.
Mai A, et al.
Journal of Medicinal Chemistry, 49(20), 6046-6056 (2006)
Synthesis and biological investigation of S-aryl-S-DABO derivatives as HIV-1 inhibitors.
Mugnaini C, et al.
Bioorganic & Medicinal Chemistry Letters, 16(13), 3541-3544 (2006)
Antithyroid Drugs and their Analogues Protect Against Peroxynitrite-Mediated Protein Tyrosine Nitration-A Mechanistic Study.
Bhabak KP and Mugesh G
Chemistry?A European Journal , 16(4), 1175-1185 (2010)
D Matthias et al.
Atherosclerosis, 122(2), 201-216 (1996-05-01)
Following oral administration of methionine in high doses to normotensive (NR) and spontaneously hypertensive (SHR) rats, its degradation product, homocysteine (HC), which is markedly elevated in serum, exerts an angiotoxic action directed to the aorta. This is accompanied by considerable
A M Attia et al.
Nucleosides & nucleotides, 18(10), 2307-2315 (2000-01-05)
N3-beta-D-glucopyranosyl, galactopyranosyl and xylopyranosyl 6-methyl-2-methylthiouracil and their 5-bromo derivatives have been synthesized by coupling an alpha-acetobromosugar with the corresponding thiouracil. The new modified thiouridine analogues were evaluated for their inhibitory activity against Human Immunodeficiency Virus (HIV) replication in MT-4 cells
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service