T89605
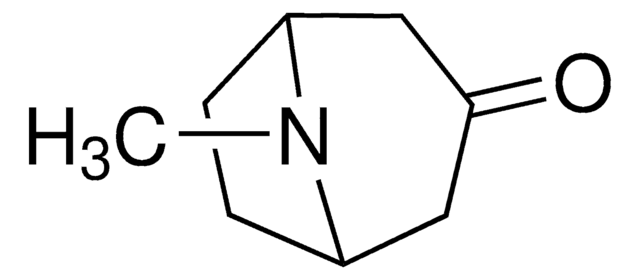
Tropinone
99%
Synonym(s):
8-Methyl-8-azabicyclo[3.2.1]octan-3-one
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H13NO
CAS Number:
Molecular Weight:
139.19
Beilstein:
2329
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
bp
113 °C/25 mmHg (lit.)
mp
40-44 °C (lit.)
storage temp.
2-8°C
SMILES string
CN1[C@@H]2CC[C@H]1CC(=O)C2
InChI
1S/C8H13NO/c1-9-6-2-3-7(9)5-8(10)4-6/h6-7H,2-5H2,1H3/t6-,7+
InChI key
QQXLDOJGLXJCSE-KNVOCYPGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
194.0 °F - closed cup
Flash Point(C)
90 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Portsteffen et al.
Phytochemistry, 37(2), 391-400 (1994-09-01)
In tropane-alkaloid producing plants and root cultures, the reduction of tropinone is a branch-point in secondary metabolism. Two different reductases stereospecifically form the isomeric alcohols tropine (tropan-3 alpha-ol) and pseudotropine (tropan-3 beta-ol). We describe here the purification and characterization of
R Xu et al.
Journal of medicinal chemistry, 41(17), 3220-3231 (1998-08-14)
A number of O-alkynyloximes of tropinone and N-methyl-4-piperidinone have been synthesized and evaluated for muscarinic activity. The affinities of these oximes were tested in homogenates of cerebral cortex, heart, and submandibulary glands from rats using [3H]pirenzepine and [3H]-N-methylscopolamine as radioligands.
Andrea Brock et al.
The Plant journal : for cell and molecular biology, 54(3), 388-401 (2008-01-29)
Tropane alkaloids typically occur in the Solanaceae and are also found in Cochlearia officinalis, a member of the Brassicaceae. Tropinone reductases are key enzymes of tropane alkaloid metabolism. Two different tropinone reductases form one stereoisomeric product each, either tropine for
Tropane alkaloid biosynthesis. A century old problem unresolved.
A J Humphrey et al.
Natural product reports, 18(5), 494-502 (2001-11-09)
Solid-phase synthesis of tertiary N-methyl amines including tropanes.
Michal Sienkiewicz et al.
Journal of combinatorial chemistry, 12(1), 5-8 (2009-11-03)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service