QBD10247
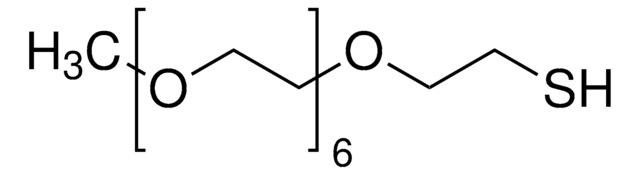
Thiol-dPEG®4-acid
>95% (HPLC)
Synonym(s):
Carboxy-PEG4-thiol, HS-PEG4-COOH, Thiol-PEG-acid, Thiol-PEG4-COOH, Thiol-PEG4-acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H22O6S
Molecular Weight:
282.35
MDL number:
UNSPSC Code:
12352106
NACRES:
NA.22
Recommended Products
Assay
>95% (HPLC)
form
solid or viscous liquid
reaction suitability
reaction type: Pegylations
polymer architecture
shape: linear
functionality: heterobifunctional
shipped in
ambient
storage temp.
−20°C
Features and Benefits
Thiol-dPEG4-acid is a sulfhydryl-containing, crosslinking PEGylation reagent that has a cross-bridge of monodisperse polyethylene glycol. The single molecular weight, discrete-length PEG (dPEG) chain is 16 atoms (18.3 Å) long. The sulfhydryl end of the crosslinker reacts with gold (forming dative bonds) and with thiol-reactive functional groups such as maleimide, bromoacetyl, SPDP, and thiol. The carboxylic acid end of the molecule couples to free amines using EDC or any suitable carbodiimide. An acylating agent such as N-hydroxysuccinimide (NHS) or 2,3,5,6-tetrafluorophenol (TFP) can enhance coupling efficiency. The carboxylate must react after the sulfhydryl conjugation.
Legal Information
Products Protected under U.S. Patent #s 7,888,536 & 8,637,711 and European Patent #s 1,594,440 & 2,750,681
dPEG is a registered trademark of Quanta BioDesign
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pi-Chou Hsieh et al.
BioMed research international, 2017, 5041683-5041683 (2017-05-02)
Herein, we report a method of combining bioinformatics and biosensing technologies to select aptamers against prostate specific antigen (PSA). The main objective of this study is to select DNA aptamers with higher binding affinity for PSA by using the proposed
Daichi Okuno et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 32(12), 1353-1357 (2016-12-13)
The artificial bilayer single-channel recording technique is commonly used to observe detailed pharmacological properties of various ion channel proteins. It permits easy control of the solution and membrane lipid composition, and is also compatible with pharmacological screening devices. However, its
Carrie A Simpson et al.
ACS nano, 5(5), 3577-3584 (2011-04-09)
Monolayer-protected gold nanoparticles have great potential as novel building blocks for the design of new drugs and therapeutics based on the easy ability to multifunctionalize them for biological targeting and drug activity. In order to create nanoparticles that are biocompatible
Daichi Okuno et al.
Nanoscale, 10(8), 4036-4040 (2018-02-13)
The artificial bilayer single channel recording technique is commonly used to observe the detailed physiological properties of various ion channel proteins. It permits easy control of the solution and membrane lipid composition, and is also compatible with pharmacological screening devices.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![O-(3-Carboxypropyl)-O′-[2-(3-mercaptopropionylamino)ethyl]-polyethylene glycol Mw 3000](/deepweb/assets/sigmaaldrich/product/structures/271/277/d02536f0-83d7-4416-9cea-f6213e09fe85/640/d02536f0-83d7-4416-9cea-f6213e09fe85.png)
