N15553
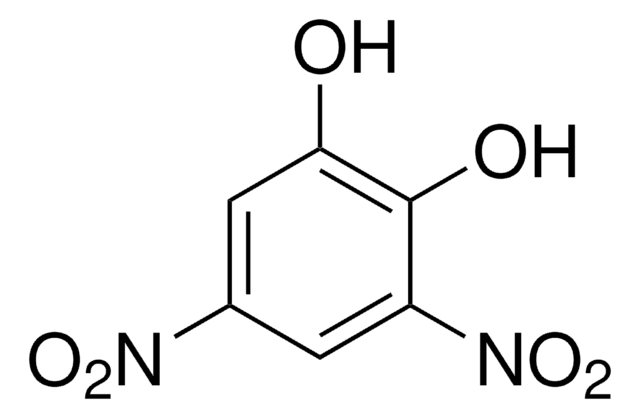
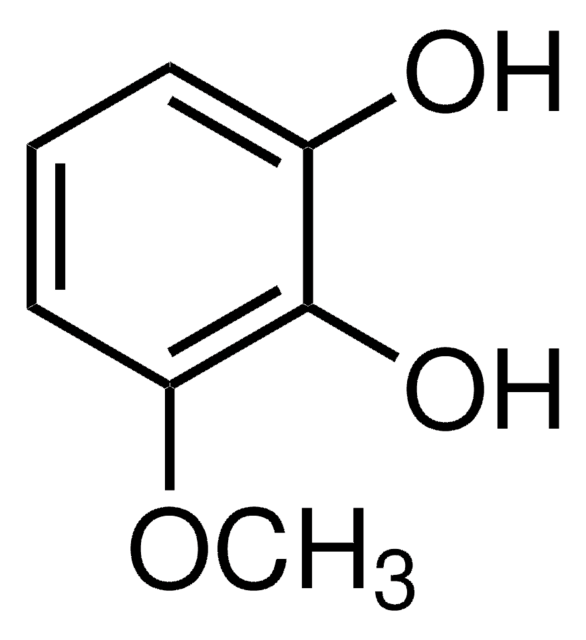
4-Nitrocatechol
≥96.0%
Synonym(s):
1,2-Dihydroxy-4-nitrobenzene, 4-Nitropyrocatechol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H3-1,2-(OH)2
CAS Number:
Molecular Weight:
155.11
Beilstein:
1867508
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥96.0%
form
powder
mp
173-177 °C (lit.)
SMILES string
Oc1ccc(cc1O)[N+]([O-])=O
InChI
1S/C6H5NO4/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3,8-9H
InChI key
XJNPNXSISMKQEX-UHFFFAOYSA-N
Gene Information
rat ... Nos1(24598)
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ayelet Fishman et al.
Biotechnology and bioengineering, 87(6), 779-790 (2004-08-27)
After discovering that toluene 4-monooxygenase (T4MO) of Pseudomonas mendocina KR1 oxidizes nitrobenzene to 4-nitrocatechol, albeit at a very low rate, this reaction was improved using directed evolution and saturation mutagenesis. Screening 550 colonies from a random mutagenesis library generated by
C Shen et al.
British journal of pharmacology, 153(4), 784-791 (2007-12-12)
Rifampicin has been extensively reported to exacerbate the hepatotoxicity of isoniazid in patients with tuberculosis. However, this was controversially claimed by previous reports using rat models. This study evaluated the effect of rifampicin on isoniazid-induced hepatocyte toxicity by using human
Yongjun Liu et al.
Journal of hazardous materials, 181(1-3), 1010-1015 (2010-06-26)
Liquid-phase decomposition of 4-nitrophenol (4-NP) and formation of hydrogen peroxide (H(2)O(2)) induced by contact glow discharge electrolysis (CGDE) were investigated. Experimental results showed that the decays of 4-NP and total organic carbon (TOC) obeyed the first-order and pseudo-first-order reaction kinetics
A Chauhan et al.
Journal of applied microbiology, 88(5), 764-772 (2000-05-03)
Pseudomonas cepacia RKJ200 (now described as Burkholderia cepacia) has been shown to utilize p-nitrophenol (PNP) as sole carbon and energy source. The present work demonstrates that RKJ200 utilizes 4-nitrocatechol (NC) as the sole source of carbon, nitrogen and energy, and
Mark F Reynolds et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 8(3), 263-272 (2003-02-18)
Mn(II)-dependent 3,4-dihydroxyphenylacetate 2,3-dioxygenase (MndD) is an extradiol-cleaving catechol dioxygenase from Arthrobacter globiformis that has 82% sequence identity to and cleaves the same substrate (3,4-dihydroxyphenylacetic acid) as Fe(II)-dependent 3,4-dihydroxyphenylacetate 2,3-dioxygenase (HPCD) from Brevibacterium fuscum. We have observed that MndD binds the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service