M7707
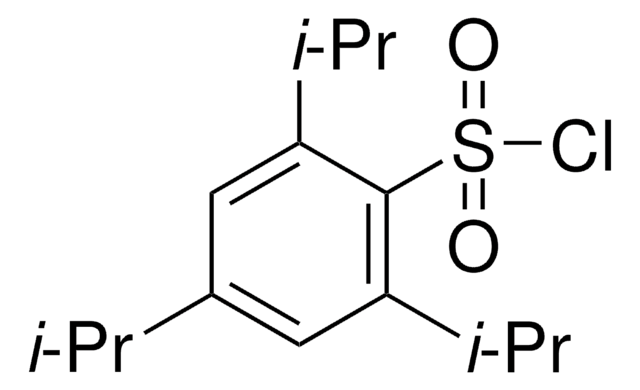
2-Mesitylenesulfonyl chloride
99%
Synonym(s):
2,4,6-Trimethylbenzenesulfonyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3C6H2SO2Cl
CAS Number:
Molecular Weight:
218.70
Beilstein:
1107601
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
mp
55-57 °C (lit.)
SMILES string
Cc1cc(C)c(c(C)c1)S(Cl)(=O)=O
InChI
1S/C9H11ClO2S/c1-6-4-7(2)9(8(3)5-6)13(10,11)12/h4-5H,1-3H3
InChI key
PVJZBZSCGJAWNG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Mesitylenesulfonyl chloride, also known as 2,4,6-Trimethylbenzenesulfonyl chloride, is an organic compound that is commonly used as a coupling agent in the nucleophilic substitution reaction to synthesize novel phospholipid derivatives of botulin and cyclodextrin.
Application
Coupling reagent in polynucleotide synthesis.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A family of single-isomer, dicationic cyclodextrin chiral selectors for capillary electrophoresis: M ono-6A-ammonium-6C-butylimidazolium-beta-cyclodextrin chlorides
Yun D, et al.
Electrophoresis, 34, 833-840 (2013)
Stéphane Menuel et al.
Beilstein journal of organic chemistry, 16, 2598-2606 (2020-11-03)
The mechanically assisted synthesis of organic compounds has recently focused considerable attention as it may be unique in features to selectively direct the reaction pathway. In the continuation of our work on the synthesis of modified cyclodextrins (CDs) via mechanochemical
Tetrahedron, 48, 1729-1729 (1992)
Synthesis of 28-O-(1, 2-Diacyl-SN-glycero-3-phospho)-betulin
B Tubek, et al.
Synthetic Communications, 42, 3648-3654 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service