M59203
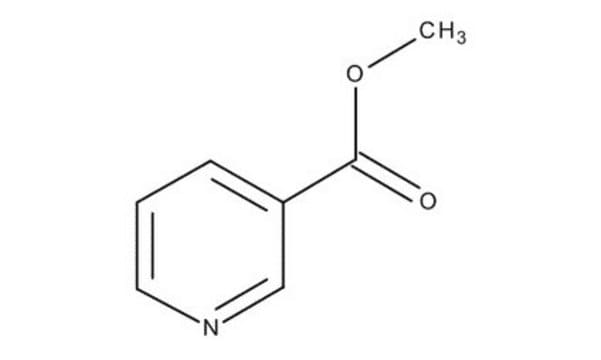
Methyl nicotinate
99%
Synonym(s):
Nicotinic acid methyl ester
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C7H7NO2
CAS Number:
Molecular Weight:
137.14
Beilstein:
113951
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
biological source
synthetic
Quality Level
Assay
99%
form
powder or crystals (possibly with chunks)
color
white to faint yellow
bp
204 °C (lit.)
mp
42-44 °C (lit.)
SMILES string
COC(=O)c1cccnc1
InChI
1S/C7H7NO2/c1-10-7(9)6-3-2-4-8-5-6/h2-5H,1H3
InChI key
YNBADRVTZLEFNH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methyl nicotinate (or nicotinic acid methyl ester) is used as a rubefacient for the relief of pains in muscles, tendons, and joints. It is also used in food as a flavoring agent.
Application
Methyl nicotinate can be employed as a precursor in the synthesis:
- Di-3-pyridyl ketone ligand, which is used in the preparation of silver(I) complexes for the derivation of coordination polymeric chains.
- 5-arylnicotinates , ±-sesbanine , and 1,4-dihydropyridine derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 3, 5-diacyl-4-phenyl-1, 4-dihydropyridines
Bennasar M-L, et al.
Tetrahedron Letters, 39(50), 9275-9278 (1998)
Helical and zigzag coordination polymeric chains derived from di-3-pyridyl ketone and silver (I) salts
Chen X-D and Mak Thomas CW
Journal of Molecular Structure, 743(1-3), 1-6 (2005)
A facile synthesis of ? -sesbanine via γ-addition of ketene silyl acetal with quaternized methyl nicotinate
Wada M, et al.
Tetrahedron Letters, 26(27), 3267-3270 (1985)
Topical antirheumatic agents as hydroxyl radical scavengers
Billany MR, et al.
International Journal of Pharmaceutics, 124(2), 279-283 (1995)
A general synthesis of 5-arylnicotinates
Thompson WJ and Gaudino J
The Journal of Organic Chemistry, 49(26), 5237-5243 (1984)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service