K401
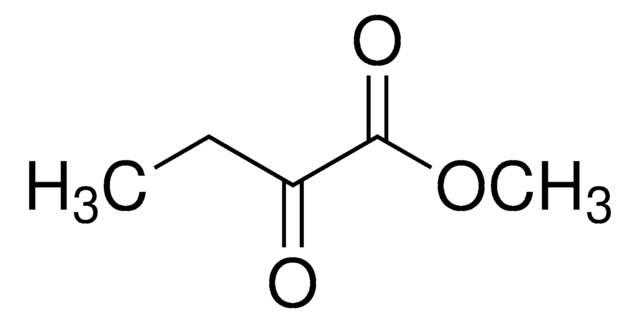
2-Ketobutyric acid
97%
Synonym(s):
2-Oxobutanoic acid, 2-Oxobutyric acid, α-Ketobutyric acid, Propionylformic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3CH2COCOOH
CAS Number:
Molecular Weight:
102.09
Beilstein:
1700514
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
bp
84 °C/20 mmHg (lit.)
mp
30-34 °C (lit.)
storage temp.
2-8°C
SMILES string
CCC(=O)C(O)=O
InChI
1S/C4H6O3/c1-2-3(5)4(6)7/h2H2,1H3,(H,6,7)
InChI key
TYEYBOSBBBHJIV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Ketobutyric acid can be used as a reactant to synthesize:
- (S)-2-Aminobutyric acid via enzyme-catalyzed amination reaction.
- L-Isoleucine by living cell reaction in the presence of ethanol.
- 3-Ethyl-2-quinoxalinol from 1,2-diaminobenzene in the presence of sulfuric acid.
- 3-Ethylbenzo[g]quinoxalin-2(1H)-one using 2,3-diaminonaphthalene in the presence of biocatalyst.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 2-(Quinoxalin-2-ylamino-benzotriazolyl) Pentanedioic Derivatives as Potential Anti-Folate Agents
Briguglio I, et al.
Journal of Heterocyclic Chemistry, 53, 1721-1737 (2016)
Fabrication of a microcapsule extinguishing agent with a core-shell structure for lithium-ion battery fire safety
Zhang W, et al.
Materials Advances, 2, 4634-4642 (2021)
Synthesis of potential chemotherapic quinoxalinone derivatives by biocatalysis or microwave-assisted Hinsberg reaction
Gris J, et al.
Tetrahedron Letters, 49, 1053-1056 (2008)
Biorefinery applications of Corynebacterium glutamicum
Jojima T, et al.
Corynebacterium glutamicum, 149-172 (2013)
Saad S El-Maraghy et al.
Heliyon, 6(7), e04384-e04384 (2020-07-17)
There is increasing evidence that rhizosphere microbes contribute to the stress mitigation process, but the mechanisms of this plant-microbe interaction are not yet understood. Siderophores-producing microorganisms have been considered important for enhancing metal tolerance in plants. In this study, rhizosphere
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service