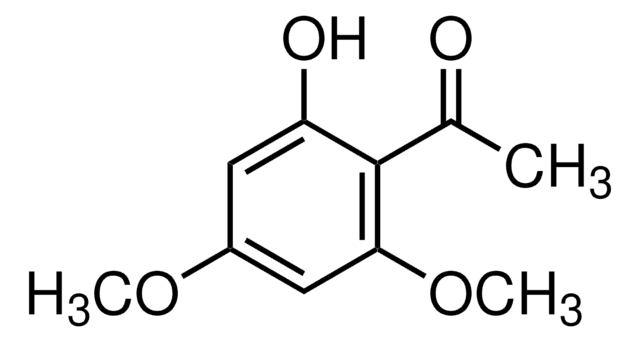
H35803
2′-Hydroxy-4′-methoxyacetophenone
99%
Synonym(s):
Paeonol, Resacetophenone 4-O-methyl ether
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOC6H3(OCH3)COCH3
CAS Number:
Molecular Weight:
166.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
48-50 °C (lit.)
SMILES string
O=C(C)C1=CC=C(OC)C=C1O
InChI
1S/C9H10O3/c1-6(10)8-4-3-7(12-2)5-9(8)11/h3-5,11H,1-2H3
InChI key
UILPJVPSNHJFIK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
In vitro and in vivo evaluation of ibuprofen-paeonol conjugate.
Dan Wu et al.
Journal of controlled release : official journal of the Controlled Release Society, 152 Suppl 1, e98-100 (2011-12-27)
Shu-zhi Zhong et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 37(17), 2603-2606 (2012-12-15)
To investigate the protective effect of paeonol on amyloid beta1-42 (Abeta1-42)-induced neurotoxicity and its mechanism. Hippocampal neurons of well-grown newborn SD rats and differentiated SH-SY5Y cell lines were cultured with various concentrations of paeonol (1, 5, 10 micromol x L(-1)
Chien-Shan Cheng et al.
Cancer management and research, 12, 641-651 (2020-02-27)
Paeonol, a natural product derived from the root of Cynanchum paniculatum (Bunge) K. Schum and the root of Paeonia suffruticosa Andr. (Ranunculaceae) has attracted extensive attention for its anti-cancer proliferation effect in recent years. The present study examined the role
Ji-Yong Liu et al.
Yao xue xue bao = Acta pharmaceutica Sinica, 47(2), 244-249 (2012-04-19)
Investigation of the pharmacokinetics of paeonol microemulsion, microemulsion-based gels and marketed paeonol ointments by the skin-blood synchronous microdialysis coupled with LC/MS is reported in this study. The microdialysis systems were established by linear probes and concentric circles probes. In vivo
Yue-Qin Wang et al.
Biological & pharmaceutical bulletin, 35(5), 767-772 (2012-06-13)
Atherosclerosis is a chronic inflammatory disease characterized by increased expression of adhesion molecules, which contribute to monocytes adhesion to vascular endothelial cells (VECs). Paeonol, an active compound isolated from cortex Moutan, has been shown to have therapeutic effects on atherosclerotic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service