C99000
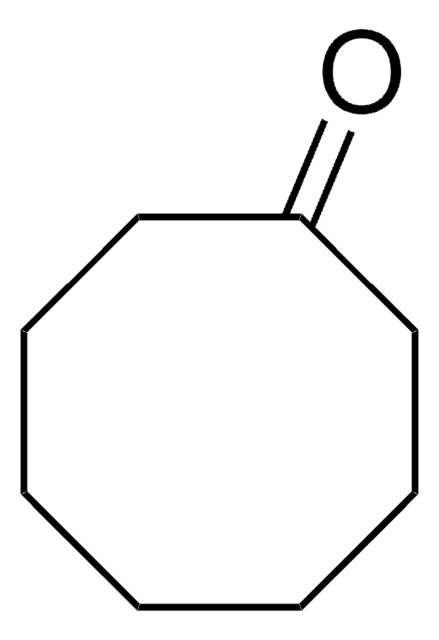
Cycloheptanone
99%
Synonym(s):
Ketocycloheptane, Ketoheptamethylene, Suberone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C7H12(=O)
CAS Number:
Molecular Weight:
112.17
Beilstein:
969823
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.461 (lit.)
bp
179 °C (lit.)
density
0.951 g/mL at 25 °C (lit.)
SMILES string
O=C1CCCCCC1
InChI
1S/C7H12O/c8-7-5-3-1-2-4-6-7/h1-6H2
InChI key
CGZZMOTZOONQIA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
131.0 °F
Flash Point(C)
55 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Brian T Sullivan et al.
Journal of chemical ecology, 47(1), 10-27 (2021-01-07)
We investigated geographic variation in the semiochemistry of major disturbance agents of western North American pine forests, Dendroctonus brevicomis Le Conte and Dendroctonus barberi Hopkins (Coleoptera: Curculionidae: Scolytinae), species separated by the Great Basin in the USA that until recently
Redouane Beniazza et al.
The Journal of organic chemistry, 76(3), 791-799 (2011-01-13)
A short access to homocalystegine analogues silylated at C7 is described. The synthesis involves the desymmetrization of a (phenyldimethylsilyl)methylcycloheptatriene using osmium-mediated dihydroxylation, followed by the diol protection and a cycloaddition involving the remaining diene moiety and an acylnitroso reagent. Additions
Avidor Shulman et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 8(1), 229-239 (2002-02-02)
Antibody 38C2 efficiently catalyzes deuterium-exchange reactions at the alpha position of a variety of ketones and aldehydes, including substrates that have a variety of sensitive functional groups. In addition to the regio- and chemoselectivity of these reactions, the catalytic rates
Takuya Hashimoto et al.
Journal of the American Chemical Society, 131(18), 6614-6617 (2009-04-22)
Insertion of one methylene unit into the C-C bond of cyclohexanones is a potentially useful, straightforward method for the construction of seven-membered carbocycles. An especially appealing but largely unexplored method in this arena is the nucleophilic addition of diazoalkanes to
Khairia M Youssef et al.
Archiv der Pharmazie, 337(1), 42-54 (2004-02-05)
New series of 3, 5-bis(substituted benzylidene)-4-piperidones, 2, 7-bis(substituted benzylidene)cycloheptanones, 1, 5-bis(substituted phenyl)-1, 4-pentadien-3-ones, 1, 7-bis(substituted phenyl)-1, 6-heptadien-3, 5-diones, 1, 1-bis(substituted cinnamoyl)-cyclopentanes, and 1, 1-bis(substituted cinnamoyl)cyclohexanes have been synthesized and tested for their antioxidant activity. Among the tested compounds, compounds II(4)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service