C86006
Crotyl alcohol, mixture of cis and trans
96%
Synonym(s):
2-Buten-1-ol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
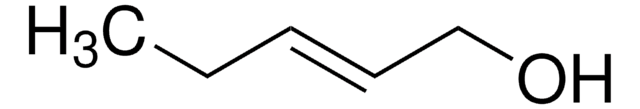
CH3CH=CHCH2OH
CAS Number:
Molecular Weight:
72.11
Beilstein:
1361395
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.427 (lit.)
bp
121-122 °C (lit.)
density
0.845 g/mL at 25 °C (lit.)
SMILES string
C\C=C\CO
InChI
1S/C4H8O/c1-2-3-4-5/h2-3,5H,4H2,1H3/b3-2+
InChI key
WCASXYBKJHWFMY-NSCUHMNNSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Crotyl alcohol can be used as a starting material in the synthesis of antitumor agents such as 14-azacamptothecin and 10, 11-methylenedioxy-14-azacamptothecin. It can also be used as a precursor in the total synthesis of discodermolide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
93.2 °F - closed cup
Flash Point(C)
34 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of optically active β-alkyl aspartate via [3, 3] sigmatropic rearrangement of α-acyloxytrialkylsilane.
Sakaguchi K, et al.
Tetrahedron Letters, 45(30), 5869-5872 (2004)
Synthesis of 14-azacamptothecin, a water-soluble topoisomerase I poison.
Rahier N J, et al.
Organic Letters, 7(5), 835-837 (2005)
Frank R Fontaine et al.
Chemical research in toxicology, 15(8), 1051-1058 (2002-08-20)
Recent confirmation that the toxic unsaturated aldehyde crotonaldehyde (CA) contributes to protein damage during lipid peroxidation confers interest on the molecular actions of this substance. However, since a plethora of structurally related aldehydes form during membrane oxidation, clarifying the toxicological
Darby R Schmidt et al.
Organic letters, 5(19), 3535-3537 (2003-09-12)
[structure: see text] A synthesis of the C(15)-C(30) fragment of Dolabelides A and B has been achieved. The recently developed asymmetric silane alcoholysis and tandem silylformylation-crotylsilylation reactions were used as the key steps to establish the C(23)-C(27) 1,5-syn-diol. In addition
Evaluation of the role of acetaldehyde in the actions of ethanol on gluconeogenesis by comparison with the effects of crotonol and crotonaldehyde.
A I Cederbaum et al.
Alcoholism, clinical and experimental research, 6(1), 100-109 (1982-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service