B78114
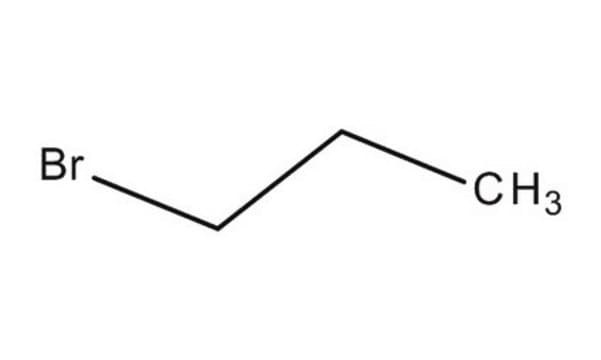
2-Bromopropane
99%
Synonym(s):
Isopropyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2CHBr
CAS Number:
Molecular Weight:
122.99
Beilstein:
741852
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.425 (lit.)
bp
59 °C (lit.)
mp
−89 °C (lit.)
density
1.31 g/mL at 25 °C (lit.)
SMILES string
CC(C)Br
InChI
1S/C3H7Br/c1-3(2)4/h3H,1-2H3
InChI key
NAMYKGVDVNBCFQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-Bromopropane is a general reagent used to introduce isopropyl group as in the case of the synthesis of various Buchwald ligands. It can also be used as a starting material in the total synthesis of lamellarin D, lamellarin H, ningalin B and (±)-dichroanal.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Repr. 1A - STOT RE 2 Inhalation
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
66.2 °F - closed cup
Flash Point(C)
19 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Total synthesis of lamellarins D, H, and R and ningalin B.
Li Q, et al.
Organic Letters, 13(2), 312-315 (2010)
An extremely active catalyst for the Negishi cross-coupling reaction.
Milne J E and Buchwald S L
Journal of the American Chemical Society, 126(40), 13028-13032 (2004)
The selective reaction of aryl halides with KOH: synthesis of phenols, aromatic ethers, and benzofurans.
Anderson K W, et al.
Journal of the American Chemical Society, 128(33), 10694-10695 (2006)
Efficient route to 4a-methyltetrahydrofluorenes: a total synthesis of (?)-dichroanal B via intramolecular Heck reaction.
Planas L, et al.
The Journal of Organic Chemistry, 71(7), 2896-2898 (2006)
NTP-CERHR Expert Panel Report on the reproductive and developmental toxicity of 2-bromopropane.
Kim Boekelheide et al.
Reproductive toxicology (Elmsford, N.Y.), 18(2), 189-217 (2004-03-17)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service