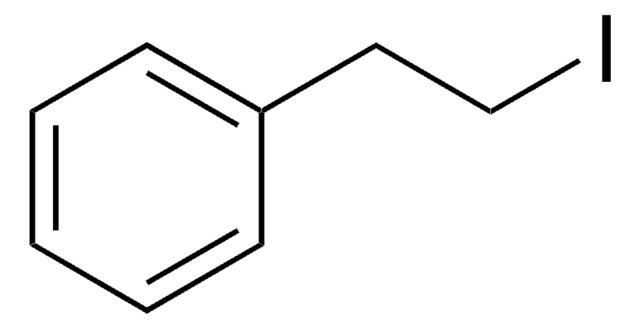
B65780
(2-Bromoethyl)benzene
98%
Synonym(s):
2-Phenylethyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2CH2Br
CAS Number:
Molecular Weight:
185.06
Beilstein:
507487
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.556 (lit.)
bp
220-221 °C (lit.)
density
1.355 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
BrCCc1ccccc1
InChI
1S/C8H9Br/c9-7-6-8-4-2-1-3-5-8/h1-5H,6-7H2
InChI key
WMPPDTMATNBGJN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(2-Bromoethyl)benzene can be used as a reactant to synthesize:
- Lactone derivatives by Sn-catalyzed double carbonylation reaction
- α-iminocarboxamides by via one-pot, three-component coupling reaction of aryl isocyanide and aryl isocyanate in the presence of SmI2.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hisashi Masui et al.
Organic letters, 14(16), 4090-4093 (2012-08-07)
A one-pot, three-component coupling was accomplished via the nucleophilic addition of an alkylsamarium(III) species to isocyanides and the subsequent addition of the resultant imidoyl samarium(III) species to isocyanates under mild conditions for the formation of α-iminocarboxamides. The developed sequential C-C
Jiang Yan et al.
Science advances, 6(45) (2020-11-08)
Phenol is an important commodity chemical in the industry, which is currently produced using fossil feedstocks. Here, we report a strategy to produce phenol from lignin by directly deconstructing Csp2-Csp3 and C-O bonds under mild conditions. It was found that
Son T Nguyen et al.
Bioorganic & medicinal chemistry, 23(9), 2024-2034 (2015-03-31)
Recently we described a novel pyranopyridine inhibitor (MBX2319) of RND-type efflux pumps of the Enterobacteriaceae. MBX2319 (3,3-dimethyl-5-cyano-8-morpholino-6-(phenethylthio)-3,4-dihydro-1H-pyrano[3,4-c]pyridine) is structurally distinct from other known Gram-negative efflux pump inhibitors (EPIs), such as 1-(1-naphthylmethyl)-piperazine (NMP), phenylalanylarginine-β-naphthylamide (PAβN), D13-9001, and the pyridopyrimidine derivatives. Here
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service