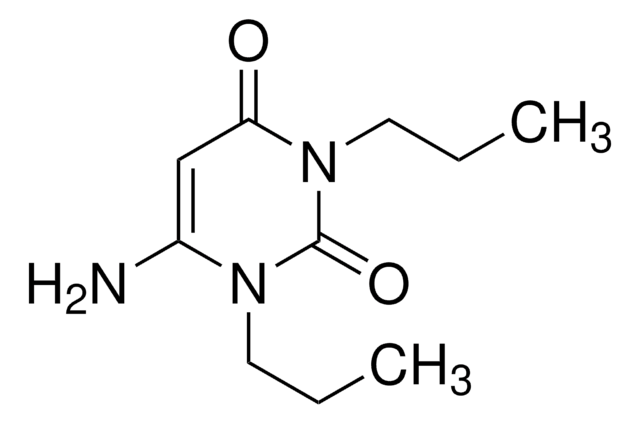
A50606
6-Aminouracil
97%
Synonym(s):
4-Amino-2,6-dihydroxypyrimidine, 6-Amino-2,4-pyrimidinediol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H5N3O2
CAS Number:
Molecular Weight:
127.10
Beilstein:
120491
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
≥360 °C (lit.)
SMILES string
Nc1cc(O)nc(O)n1
InChI
1S/C4H5N3O2/c5-2-1-3(8)7-4(9)6-2/h1H,(H4,5,6,7,8,9)
InChI key
LNDZXOWGUAIUBG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
María-Jesús Pérez-Pérez et al.
Mini reviews in medicinal chemistry, 5(12), 1113-1123 (2005-12-27)
Thymidine Phosphorylase (TPase) catalyses the reversible phosphorolysis of pyrimidine 2'-deoxynucleosides to 2-deoxyribose-1-phosphate and their respective pyrimidine bases, including the phosphorolysis of nucleoside analogues with important antiviral or anticancer properties. Moreover, TPase, identified also as the angiogenic platelet-derived endothelial cell growth
H Sladowska et al.
Acta poloniae pharmaceutica, 53(1), 39-46 (1996-01-01)
Synthesis of 6-substituted 2,4-dioxo-1,2,3,4,5,6,7,8-octahydropyrimido[4,5-d]pyrimidines [III-VI] obtained by cyclocondensation of 1-phenyl-6-aminouracil with formaline and the primary amines is described. Compounds III, V, VI in the Mannich reaction with secondary cyclic amines yield the corresponding 3-substituted N-aminomethyl derivatives VII-X. Some of them
P M Tarantino et al.
Journal of medicinal chemistry, 42(11), 2035-2040 (1999-06-04)
6-Anilinouracils (6-AUs) are dGTP analogues which selectively inhibit the DNA polymerase III of Bacillus subtilis and other Gram-positive bacteria. To enhance the potential of the 6-AUs as antimicrobial agents, a structure-activity relationship was developed involving substitutions of the uracil N3
A Maldonado et al.
Experimental cell research, 161(1), 172-180 (1985-11-01)
We have approached the study of the ability of different types of lesions produced by DNA-damaging agents to develop sister-chromatid exchanges (SCEs) by analyzing SCE levels observed in Allium cepa L cells with BrdU-substituted DNA and exposed to visible light
Akos Bányász et al.
The journal of physical chemistry. B, 114(39), 12708-12719 (2010-09-14)
A detailed experimental and computational study of the absorption and fluorescence spectra of 5-aminouracil (5 AU) and 6-aminouracil (6 AU) in aqueous solution is reported. The lowest energy band of the steady-state absorption spectra of 5 AU is considerably red-shifted
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service