910716
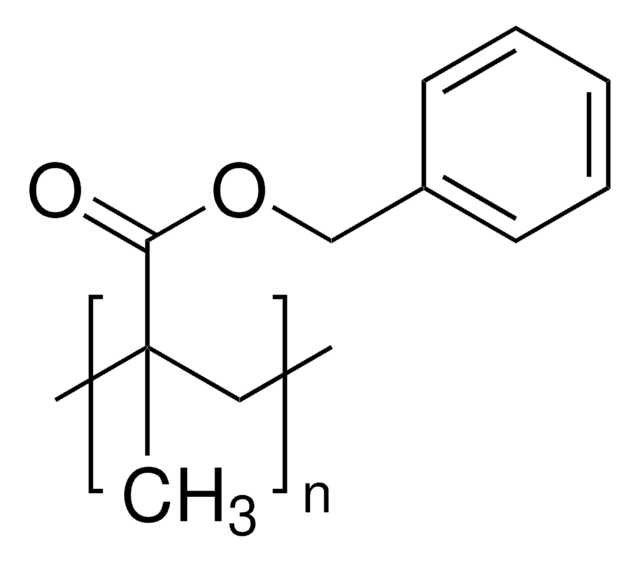
Poly(butyl methacrylate)
free flow beads, Mw 211,000
Synonym(s):
PBMA
About This Item
Recommended Products
description
Brookfield Viscosity in 40% toluene: 500-800 cPs
Glass transition temperature onset: 20 °C
Maximum % Moisture: 0.30%
form
solid (bead)
mol wt
Mw 211,000
refractive index
n/D 1.483
density
1.07 g/mL at 25 °C (lit.)
InChI
1S/C8H14O2/c1-4-5-6-10-8(9)7(2)3/h2,4-6H2,1,3H3
InChI key
SOGAXMICEFXMKE-UHFFFAOYSA-N
General description
Conventional high molecular weight poly(butyl methacrylate) easily forms a fused block that require mechanical force (such as hammering) to break it down into small particles before usage. This product was developed with an anti-blocking technology and comes in an easy-to-use, free flowing, bead form.
Application
It may be used to study the photoluminescence in EuTFC embedded in Poly(butyl methacrylate) (PBMA) polymer films.
It has also been used for coatings, surface modification, solid state battery, silk screen inks, adhesives for plastic and aluminum, plasticizer for hard butyl methacrylate resins, and for improving outdoor durability of vinyl chloride resins in pigmented lacquers.
Preparation Note
This polymer dissolves at room temperature but requires constant agitation to prevent solvent-swollen granules of polymer from forming agglomerates and sticking to the walls of the vessel.
Important: The polymer beads should be sifted directly into the vortex of the stirred solvent to speed wetting-out and dispersion. Continuous low-shear agitation for periods of 1-12 hours, depending on the grade and concentration of resin, is recommended.
After the solution appears clear in the tank, a sample should be spread out on a Leneta card or glass. After the solvent evaporates and a film forms on the card or glass, there should not be any resin seeds. If there are any seeds, the tank should be agitated further to fully dissolve the resin. Tank agitation should not be stopped (except for sampling) until the film test indicates there are no resin seeds. Any cloudiness or residue may indicate that some polymer remains undissolved. The presence of water in the system can also cause cloudiness.
Solution time can be reduced by heating; most common solvents can be heated to approximately 49°C (120°F) without the need for reflux equipment. High-shear agitation also cuts dissolving time, but requires care to avoid overheating and excessive solvent loss.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Graphene nanoribbons (GNRs) are quasi-one-dimensional narrow strips of graphene comprised of sp2-hybridized carbon atoms arranged into hexagonal honeycomb lattice configurations.
Graphene nanoribbons (GNRs) are quasi-one-dimensional narrow strips of graphene comprised of sp2-hybridized carbon atoms arranged into hexagonal honeycomb lattice configurations.
Graphene nanoribbons (GNRs) are quasi-one-dimensional narrow strips of graphene comprised of sp2-hybridized carbon atoms arranged into hexagonal honeycomb lattice configurations.
Graphene nanoribbons (GNRs) are quasi-one-dimensional narrow strips of graphene comprised of sp2-hybridized carbon atoms arranged into hexagonal honeycomb lattice configurations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service