900932
TAMRA alkyne
≥95%
Synonym(s):
Tetramethylrhodamine alkyne
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C36H41N3O8
Molecular Weight:
643.73
UNSPSC Code:
12352200
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
powder or crystals
reaction suitability
reaction type: click chemistry
storage temp.
−20°C
SMILES string
O=CNCCOCCOCCOCCOCC#C.CN(C)C1=CC=C(C(C2=C(C([O-])=O)C=CC=C2)=C(C=C3)C(O4)=CC3=[N+](C)C)C4=C1
Application
TAMRA alkyne is a red-fluorescent probe that through the alkyne group can be reacted with azides via a copper-catalyzed click reaction (CuAAC). TAMRA (tetramethylrhodamine) is a bright fluorescent probe and is compatible with various excitation sources including mercury arc, tungsten and xenon arc lamps, the 544 nm line of the Helium-Neon laser and the 532 nm green laser line.{37
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Selective imaging of Gram-negative and Gram-positive microbiotas in the mouse gut.
Wang W, et al.
Biochemistry, 56(30), 3889-3893 (2017)
Chemoselective modification of viral surfaces via bioorthogonal click chemistry.
Rubino F A, et al.
Journal of Visualized Experiments, 66 (2012)
Jacob Gubbens et al.
Chemistry & biology, 16(1), 3-14 (2009-01-28)
New lipid analogs mimicking the abundant membrane phospholipid phosphatidylcholine were developed to photocrosslink proteins interacting with phospholipid headgroups at the membrane interface. In addition to either a phenylazide or benzophenone photoactivatable moiety attached to the headgroup, the lipid analogs contained
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service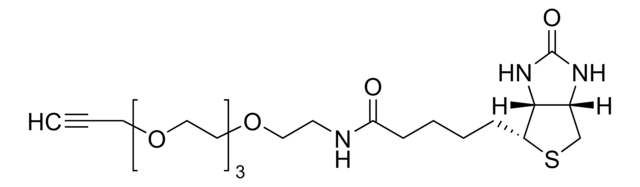








![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)
