745022
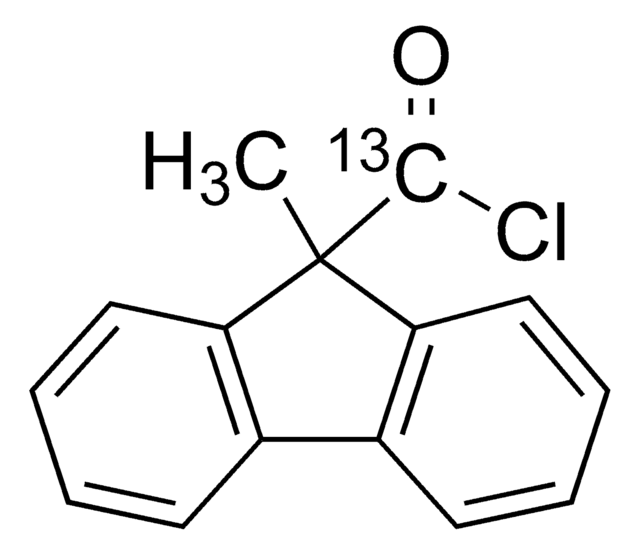
9-Methyl-9H-fluorene-9-carbonyl chloride
≥99.0% (GC)
Synonym(s):
COgen, 9-Methylfluorene-9-carbonyl chloride
About This Item
Recommended Products
Assay
≥99.0% (GC)
form
solid
reaction suitability
reaction type: C-C Bond Formation
functional group
acyl chloride
SMILES string
CC1(C(Cl)=O)c2ccccc2-c3ccccc13
InChI
1S/C15H11ClO/c1-15(14(16)17)12-8-4-2-6-10(12)11-7-3-5-9-13(11)15/h2-9H,1H3
InChI key
ZQYOOHGEBHBNTP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
For more information please visit: Technology Spotlight, Professor Skrystrup PPP
Use with the COware Platform
Linkage
also commonly purchased with this product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Studies in the field of carbonylation chemistry led to the discovery of a novel carbon monoxide (CO) delivery system.
Studies in the field of carbonylation chemistry led to the discovery of a novel carbon monoxide (CO) delivery system.
Studies in the field of carbonylation chemistry led to the discovery of a novel carbon monoxide (CO) delivery system.
Studies in the field of carbonylation chemistry led to the discovery of a novel carbon monoxide (CO) delivery system.
Related Content
The Skrydstrup group has developed reagents and glassware for carrying out transition metal catalyzed carbonylations in a simple and safe manner.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service