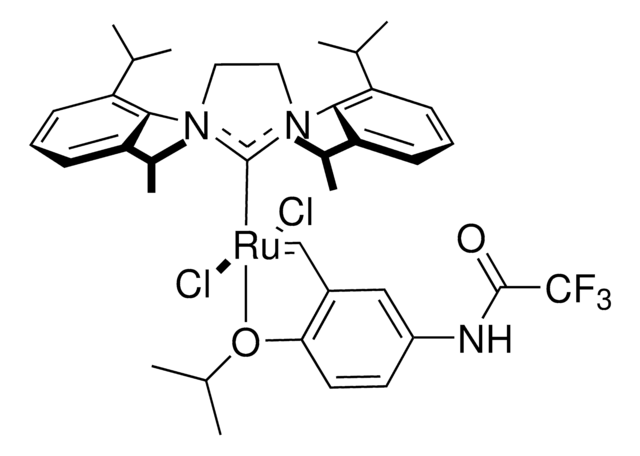
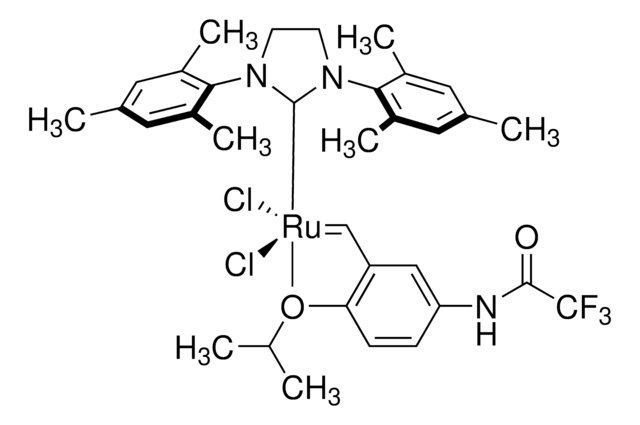
682373
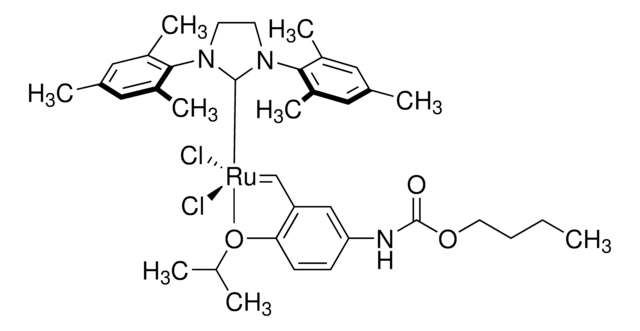
Hoveyda-Grubbs Catalyst® M721
Umicore
Synonym(s):
Hoveyda-Grubbs Catalyst® M72 SI(o-Tol) (C571), Dichloro[1,3-bis(2-methylphenyl)-2-imidazolidinylidene](2-isopropoxyphenylmethylene)ruthenium(II), Stewart-Grubbs catalyst
About This Item
Recommended Products
Quality Level
reaction suitability
core: ruthenium
reagent type: catalyst
reaction type: Ring-Opening Polymerization
mp
199-208 °C
storage temp.
2-8°C
SMILES string
CC(C)Oc1ccccc1C=[Ru](Cl)(Cl)=C2N(CCN2c3ccccc3C)c4ccccc4C
InChI
1S/C17H18N2.C10H12O.2ClH.Ru/c1-14-7-3-5-9-16(14)18-11-12-19(13-18)17-10-6-4-8-15(17)2;1-8(2)11-10-7-5-4-6-9(10)3;;;/h3-10H,11-12H2,1-2H3;3-8H,1-2H3;2*1H;/q;;;;+2/p-2
InChI key
ZDJCARGGZRTYFH-UHFFFAOYSA-L
Related Categories
General description
Application
Legal Information
Product License
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at http://www.pmc.umicore.com
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Research in the Grubbs group has centered on the development and application of a suite of highly active, selective, and bench stable ruthenium alkylidene complexes capable of catalyzing versatile olefin metatheses.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service