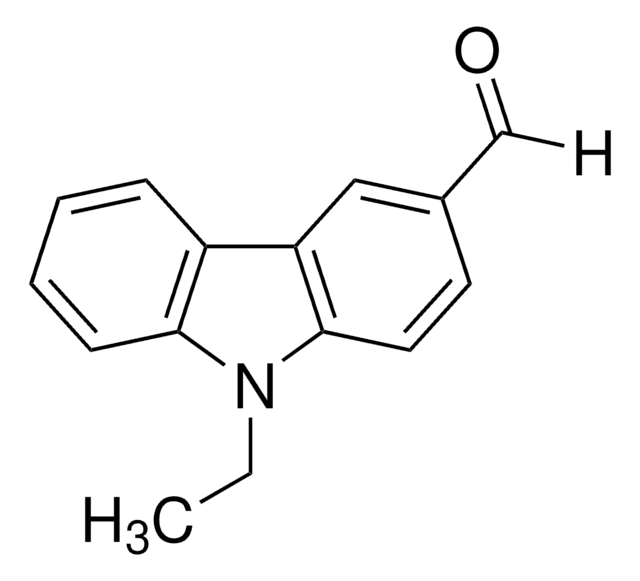
647209
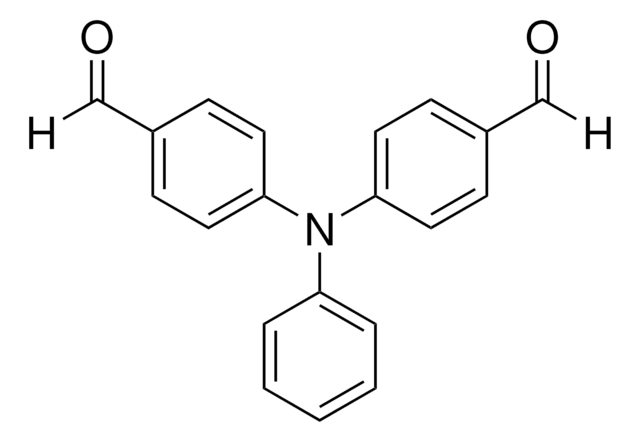
4-(Diphenylamino)benzaldehyde
97%
Synonym(s):
4-Formyltriphenylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C19H15NO
CAS Number:
Molecular Weight:
273.33
Beilstein:
2732795
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
129-133 °C (lit.)
functional group
aldehyde
SMILES string
O=Cc1ccc(cc1)N(c2ccccc2)c3ccccc3
InChI
1S/C19H15NO/c21-15-16-11-13-19(14-12-16)20(17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-15H
InChI key
UESSERYYFWCTBU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aline M Lino et al.
Physical chemistry chemical physics : PCCP, 19(31), 20984-20990 (2017-07-27)
The styryl dye (E)-2-[3-[4-(diphenylamine) phenyl]-1-(p-tolyl)-allylidene]-malononitrile (DFTAM) was prepared by Knoevenagel condensation using homogeneous and surface bound amino catalysts. The catalysis by surface bound piperazine allowed the study of the condensation reaction at a single molecule (SM) level using total internal
Viprabha Kakekochi et al.
ChemPlusChem, 85(8), 1762-1777 (2020-08-15)
A set of four symmetric, butterfly-shaped 4-(4-(decyloxy)phenyl)-2,6-di(thiophen-2-yl)pyridine (TPY) derivatives 2TPA-TPY (TPY center and triphenylamine end groups), 2CBZ-TPY (TPY center and N-ethyl carbazole end groups), 2TPY-TPA (triphenylamine center and TPY at the periphery) and 2TPY-CBZ (N-ethyl carbazole center and TPY at
Anu Kundu et al.
Journal of fluorescence, 29(6), 1359-1369 (2019-11-16)
New series of methoxy and hydroxyl group substituted triphenylamine (TPA)-imidazole fluorescent molecules (5-(diphenylamino)-2-(1H-phenanthro[9,10-d]imidazol-2-yl)phenol (1), 5-(diphenylamino)-2-(1-phenyl-1H-phenanthro[9,10-d]imidazol-2-yl)phenol (2), 5-(diphenylamino)-2-(4,5-diphenyl-1H-imidazol-2-yl)phenol (3), 5-(diphenylamino)-2-(1,4,5-triphenyl-1H-imidazol-2-yl)phenol (4), N-(3-methoxy-4-(1H-phenanthro[9,10-d]imidazol-2-yl)phenyl)-N-phenylbenzenamine (5), N-(3-methoxy-4-(1-phenyl-1H-phenanthro[9,10-d]imidazol-2-yl)phenyl)-N-phenylbenzene amine (6), and N-(3-methoxy-4-(4,5-diphenyl-1H-imidazol-2-yl)phenyl)-N-phenylbenzenamine (7)) have been synthesized that exhibited strong solution fluorescence and molecular structure and
Danuta Sek et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 175, 24-35 (2016-12-25)
The new Schiff bases bearing anthracene unit were synthesized from 2-aminoanthracene and various aldehydes such as: benzaldehyde, 4-(diphenylamino)benzaldehyde, 9-phenanthrenecarboxaldehyde, 9-anthracenecarboxaldehyde, and biphenyl-4-carboxaldehyde, 2-naphthaldehyde. Resulted azomethines were characterized by IR, NMR (
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service